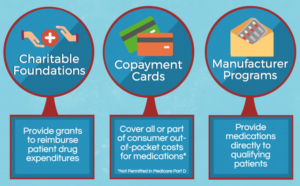
If you are uninsured or underinsured, Otezla SupportPlus can put you in touch with the Amgen Safety Net Foundation, a nonprofit patient assistance program sponsored by Amgen that helps qualifying patients access Otezla and other Amgen medicines at no cost. Starting Otezla? Start off right with your own personalized support—an Otezla Nurse Partner.
Full Answer
Do I have to buy Otezla?
No purchase necessary. Please see full Terms and Conditions. If you are uninsured or underinsured, Otezla SupportPlus can put you in touch with the Amgen Safety Net Foundation, a nonprofit patient assistance program sponsored by Amgen that helps qualifying patients access Otezla and other Amgen medicines at no cost. Starting Otezla?
How can I get Otezla If I am uninsured or underinsured?
If you are uninsured or underinsured, Otezla SupportPlus can put you in touch with the Amgen Safety Net Foundation, a nonprofit patient assistance program sponsored by Amgen that helps qualifying patients access Otezla and other Amgen medicines at no cost. Starting Otezla? Start off right with your own personalized support—an Otezla Nurse Partner.
What is the Otezla co-pay card?
The Otezla Co-Pay Card is open to patients with commercial insurance, regardless of financial need. The program is not valid for patients whose Otezla prescription is paid for in whole or in part by Medicare, Medicaid, or any other federal or state programs. It is not valid for cash-paying patients or where prohibited by law.
What is the Otezla Nurse Partner‡ Program?
The Otezla Nurse Partner ‡ program works closely with your new Otezla patients to provide tailored support, including how to get started on Otezla, maintaining a treatment routine, and guiding them to resources based on their individual goals. Enrollment is simple for your Otezla patients.
How many patients have Otezla?
What is OTezla apremilast?
What is otezla used for?
What is the sNDA for Otezla?
What are the most common adverse events of Otezla?
Does apremilast affect milk production?
Is otezla safe for pregnant women?
See 2 more
About this website

What is the Otezla bridge program?
The program provides up to a Maximum Program Benefit of assistance to reduce a patient's out-of-pocket prescription costs that Amgen will provide per patient for each calendar year, which must be applied to the Otezla patient's out-of-pocket costs (co-pay, deductible, or co-insurance).
How do you get Otezla covered?
Otezla is covered by most health plans in the US, including commercial, Medicare, and Medicaid....FINANCIAL SUPPORTPatients with commercial health insurance plans.Patients covered by Medicare and Medicaid.Patients without health insurance coverage.Underinsured patients.
Does Amgen have a patient assistance program?
The AMGEN, INC. patient assistance program offers free medication to people who otherwise cannot afford their medications. Patients must meet financial and other program specific criteria to be eligible for assistance.
What is Amgen assist?
Amgen Assist 360™ is a single place for patients, caregivers, and healthcare professionals to go to find the support, tools, and resources most important to them.*
Is Otezla a Tier 4 drug?
What drug tier is Otezla typically on? Medicare prescription drug plans typically list Otezla on Tier 5 of their formulary.
Is there a generic version of Otezla?
The US Food and Drug Administration (FDA) has granted approval for the first generic version of Otezla (apremilast) in 10, 20, and 30 mg tablets. The drug is commonly used to treat diseases such as psoriatic arthritis (PsA), Behcet's Syndrome, and plaque psoriasis.
What is Amgen first step program?
The Amgen FIRST STEP™ Program is here to help eligible commercially insured patients pay for their out-of-pocket prescription costs, including deductible, co-insurance, and co-payment.
What is Amgen edge program?
Maximum Program Benefit, Patient Benefits May Change, End or Vary Without Notice: The AmgenEdge® program provides up to a Maximum Program Benefit (based upon the medication covered by the AmgenEdge® program) to reduce a patient's out-of-pocket medication costs that Amgen will provide per patient for each calendar year, ...
How do I contact Amgen?
For product questions, to report an adverse event or safety-related issue, or to report a quality issue with a product or device, call +1 800-772-6436 (800-77-AMGEN). U.S. healthcare professionals can also visit www.amgenmedinfo.com. Local contact information by country can be found below.
How can I get Aimovig for free?
The Amgen Safety Net Foundation is an independent, nonprofit patient assistance program that provides Aimovig at no cost to qualifying patients who have a financial need and are uninsured or have insurance that excludes Aimovig.
What is Amgen safety net?
Amgen Safety Net Foundation (ASNF) is an independent nonprofit patient assistance program established in 2001, sponsored by Amgen. ASNF was designed to assist patients who have a financial need, and are uninsured or the insurance plan excludes the Amgen medicine.
Is there a patient assistance program for Repatha?
RepathaReady® offers resources and support services to help patients stay on track with their high LDL treatment. Sign up today to see if you are eligible for the Repatha® Copay Card, and to receive nurse support, needle disposal containers, medication reminders and informational emails, and insurance assistance.
Is Otezla considered a specialty drug?
Otezla (apremilast) is an expensive medicine that you may only be able to get from a specialty pharmacy. You may need to be followed more closely by a healthcare provider for safety reasons. Otezla is not a biologic drug treatment.
Is Otezla hard to get?
Getting Otezla shouldn't be difficult. If you don't hear from your specialty pharmacy within a week after your doctor submitted your prescription, be sure to give Otezla SupportPlus a call at 1-844-4OTEZLA (1-844-468-3952). *The Otezla Co-Pay Card is for eligible commercially insured patients.
Does Otezla go to a specialty pharmacy?
Otezla is delivered by a specialty pharmacy. What does that mean? Unlike a retail pharmacy, where you go to pick up your medication, specialty pharmacies deliver your Otezla straight to your door.
What psoriasis medication does Medicare cover?
Medicare Coverage for Humira. Humira is biologic drug that's used to treat inflammatory conditions like rheumatoid arthritis, plaque psoriasis, and Crohn's disease. Humira is mainly covered by Medicare Part D and Part C; in rare cases, Part B may also offer coverage.
How many patients have Otezla?
Since its initial FDA approval in 2014, Otezla has been prescribed to more than 250,000 patients with moderate-to-severe plaque psoriasis or active psoriatic arthritis in the U.S. 1. About ADVANCE (PSOR-022)
What is OTezla apremilast?
About Otezla® (apremilast) OTEZLA ® (apremilast) is an oral small-molecule inhibitor of phosphodiesterase 4 (PDE4) specific for cyclic adenosine monophosphate (cAMP). PDE4 inhibition results in increased intracellular cAMP levels, which is thought to indirectly modulate the production of inflammatory mediators.
What is otezla used for?
Otezla is indicated for the treatment of adult patients with active psoriatic arthritis. Otezla is indicated for the treatment of adult patients with oral ulcers associated with Behçet's Disease. Otezla® (apremilast) U.S. IMPORTANT SAFETY INFORMATION.
What is the sNDA for Otezla?
THOUSAND OAKS, Calif., Feb. 22, 2021 /PRNewswire/ -- Amgen (NASDAQ:AMGN) today announced the submission of a supplemental New Drug Application (sNDA) to the U.S. Food and Drug Administration (FDA) for Otezla ® (apremilast) for the treatment of adults with mild-to-moderate plaque psoriasis who are candidates for phototherapy or systemic therapy. The sNDA is based on data from the Phase 3 ADVANCE trial that demonstrated oral Otezla 30 mg twice daily achieved a statistically significant improvement in the primary endpoint of the static Physician's Global Assessment (sPGA) response at week 16 compared to placebo.
What are the most common adverse events of Otezla?
The most commonly reported adverse events that occurred in at least 5% of patients in either treatment group were diarrhea, headache, nausea, nasopharyngitis and upper respiratory tract infection. Detailed results will be submitted for presentation at an upcoming medical meeting. In the U.S., Otezla is approved for the treatment ...
Does apremilast affect milk production?
Lactation: There are no data on the presence of apremilast or its metabolites in human milk, the effects of apremilast on the breastfed infant, or the effects of the drug on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Otezla and any potential adverse effects on the breastfed child from Otezla or from the underlying maternal condition
Is otezla safe for pregnant women?
Pregnancy: Otezla has not been studied in pregnant women. Advise pregnant women of the potential risk of fetal loss. Consider pregnancy planning and prevention for females of reproductive potential. There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to Otezla during pregnancy. Information about the registry can be obtained by calling 1-877-311-8972 or visiting https://mothertobaby.org/ongoing-study/otezla/
What is the Amgen Safety Net Foundation?
Since 2001, the Amgen Safety Net Foundation (ASNF) has helped hundreds of thousands of patients with financial need gain access to Amgen medicines at no cost. This is a modal window.
Does Amgen charge for patient assistance?
Check the status of your patient's application. Provider Access. Amgen Safety Net Foundation does not charge patients a fee for its assistance. Amgen Safety Net Foundation is not affiliated with third parties who charge a fee for assistance with enrollment or medication refills.
Is Amgen Safety Net Foundation billed monthly?
If you are being charged a monthly fee for support from the Amgen Safety Net Foundation, the organization billing you is not the Amgen Safety Net Foundation and you are being charged for support that the Amgen Safety Net Foundation can provide to you directly at no cost.
What is otezla used for?
Otezla ® (apremilast) is indicated for the treatment of adult patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy.
Can Otezla cause vomiting?
Diarrhea, Nausea, and Vomiting: Cases of severe diarrhea, nausea, and vomiting were associated with the use of Otezla. Most events occurred within the first few weeks of treatment. In some cases patients were hospitalized. Patients 65 years of age or older and patients taking medications that can lead to volume depletion or hypotension may be at a higher risk of complications from severe diarrhea, nausea, or vomiting. Monitor patients who are more susceptible to complications of diarrhea or vomiting; advise patients to contact their healthcare provider. Consider Otezla dose reduction or suspension if patients develop severe diarrhea, nausea, or vomiting
Is otezla safe for pregnant women?
Pregnancy: Otezla has not been studied in pregnant women. Advise pregnant women of the potential risk of fetal loss. Consider pregnancy planning and prevention for females of reproductive potential. There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to Otezla during pregnancy. Information about the registry can be obtained by calling 1-877-311-8972 or visiting https://mothertobaby.org/ongoing-study/otezla/
How many patients have Otezla?
Since its initial FDA approval in 2014, Otezla has been prescribed to more than 250,000 patients with moderate-to-severe plaque psoriasis or active psoriatic arthritis in the U.S. 1. About ADVANCE (PSOR-022)
What is OTezla apremilast?
About Otezla® (apremilast) OTEZLA ® (apremilast) is an oral small-molecule inhibitor of phosphodiesterase 4 (PDE4) specific for cyclic adenosine monophosphate (cAMP). PDE4 inhibition results in increased intracellular cAMP levels, which is thought to indirectly modulate the production of inflammatory mediators.
What is otezla used for?
Otezla is indicated for the treatment of adult patients with active psoriatic arthritis. Otezla is indicated for the treatment of adult patients with oral ulcers associated with Behçet's Disease. Otezla® (apremilast) U.S. IMPORTANT SAFETY INFORMATION.
What is the sNDA for Otezla?
THOUSAND OAKS, Calif., Feb. 22, 2021 /PRNewswire/ -- Amgen (NASDAQ:AMGN) today announced the submission of a supplemental New Drug Application (sNDA) to the U.S. Food and Drug Administration (FDA) for Otezla ® (apremilast) for the treatment of adults with mild-to-moderate plaque psoriasis who are candidates for phototherapy or systemic therapy. The sNDA is based on data from the Phase 3 ADVANCE trial that demonstrated oral Otezla 30 mg twice daily achieved a statistically significant improvement in the primary endpoint of the static Physician's Global Assessment (sPGA) response at week 16 compared to placebo.
What are the most common adverse events of Otezla?
The most commonly reported adverse events that occurred in at least 5% of patients in either treatment group were diarrhea, headache, nausea, nasopharyngitis and upper respiratory tract infection. Detailed results will be submitted for presentation at an upcoming medical meeting. In the U.S., Otezla is approved for the treatment ...
Does apremilast affect milk production?
Lactation: There are no data on the presence of apremilast or its metabolites in human milk, the effects of apremilast on the breastfed infant, or the effects of the drug on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Otezla and any potential adverse effects on the breastfed child from Otezla or from the underlying maternal condition
Is otezla safe for pregnant women?
Pregnancy: Otezla has not been studied in pregnant women. Advise pregnant women of the potential risk of fetal loss. Consider pregnancy planning and prevention for females of reproductive potential. There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to Otezla during pregnancy. Information about the registry can be obtained by calling 1-877-311-8972 or visiting https://mothertobaby.org/ongoing-study/otezla/