Full Answer
What is the Bayer Patient Assistance Program?
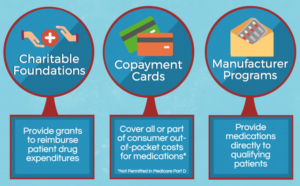
Patient Assistance Program The Bayer US Patient Assistance Foundation is a charitable organization established to assist patients who face financial challenges. Bayer believes you should be able to get the medicines you need — even if you don’t have insurance or are underinsured. Visit Patient Assistance Website
What is the Stivarga co-pay program?
Stivarga Co-Pay Program: Eligible commercially insured patients may pay $0 per prescription with savings of up to $25,000 per year; for additional information contact the program at 647-245-5622. Form more information phone: 647-245-5622 or Visit website
What is Stivarga used to treat?
Stivarga Prices, Coupons and Patient Assistance Programs. Stivarga (regorafenib) is a member of the multikinase inhibitors drug class and is commonly used for Colorectal Cancer, Gastrointestinal Stromal Tumor and Hepatocellular Carcinoma.
How much does Stivarga cost?
The cost for Stivarga oral tablet 40 mg is around $21,546 for a supply of 84, depending on the pharmacy you visit. Prices are for cash paying customers only and are not valid with insurance plans. This Stivarga price guide is based on using the Drugs.com discount card which is accepted at most U.S. pharmacies.
What is stivarga used for?
How long after stivarga can you breastfeed?
Can you die from stiverga?
Does stiverga cause diarrhea?
Can you die from taking stivarga?
Does stivarga cause infections?
See 3 more

Does Medicare pay for stivarga?
Do Medicare prescription drug plans cover Stivarga? Yes. 100% of Medicare prescription drug plans cover this drug.
How successful is stivarga?
Improvement in Overall Survival Patients taking STIVARGA (379) lived a median of 10.6 months compared to those taking placebo (194) who lived a median of 7.8 months. There were 233 deaths out of 379 patients with STIVARGA (62%) vs 140 deaths out of 194 patients with placebo (72%)
Who is the manufacturer of stivarga?
Stivarga (Regorafenib) is an oral multikinase inhibitor developed by Bayer HealthCare for the treatment of metastatic colorectal cancer (mCRC).
Why do pharmaceutical companies offer patient assistance programs?
They increase demand, allow companies to charge higher prices, and provide public-relations benefits. Assistance programs are an especially attractive proposition for firms that sell particularly costly drugs. Faced with high out-of-pocket costs, some patients may decide against taking an expensive medication.
Is Stivarga a last resort?
The drug regorafenib (Stivarga®) was approved last year as third line treatment for patients with advanced Gastrointestinal Stromal Tumour (GIST).
Does Stivarga shrink tumors?
Regorafenib may shrink the cancer or stop it growing for a time. You usually take regorafenib for as long as it is helping you and the side effects aren't too bad.
How long has STIVARGA been around?
Regorafenib demonstrated to increase the overall survival of patients with metastatic colorectal cancer and has been approved by the US FDA on September 27, 2012.
Is STIVARGA chemotherapy?
Stivarga® is the trade name for the generic chemotherapy drug regorafenib. In some cases, health care professionals may use the generic name regorafenib when referring to the trade name Stivarga®. Drug type: Stivarga® is a targeted therapy.
Does STIVARGA cause hair loss?
Weight loss. A change in voice (often described by patients as hoarseness)...Laboratory Test Abnormalities.Side effects reported in phase III trial of regorafenib vs. placeboRegorafenib (n=131)Any grade (all severities)Placebo (n=66)Any gradeAlopecia (hair loss)24%2%Rash, maculopapular18%3%Nausea16%9%10 more rows•Dec 14, 2012
What happens if you can't afford a prescription?
Your Access to Prescription and Healthcare Savings The first place to look for help are the drug patient assistance programs (PAPs). These are programs run by drug companies that give free medicine to people who can't afford to pay for them. Not everyone qualifies, but millions of people have been helped.
Is patient assistance program legitimate?
Patient assistance programs (PAPs) are usually sponsored by pharmaceutical manufacturers and are promoted as a safety net for Americans who have no health insurance or are underinsured.
How do patient support programs work?
A patient assistance or support programs (PAPs or PSPs) exist to get you timely access to medication and to help you stay on track of your therapy. Being diagnosed with a complex disease or condition may come with unexpected financial burden and a need to better understand treatment options and next steps.
Is stivarga chemotherapy?
Stivarga® is the trade name for the generic chemotherapy drug regorafenib. In some cases, health care professionals may use the generic name regorafenib when referring to the trade name Stivarga®. Drug type: Stivarga® is a targeted therapy.
What are the side effects of stivarga?
STIVARGA may cause serious side effects, including:Infection.severe bleeding.a tear in your stomach or intestinal wall (bowel perforation).a skin problem called hand-foot skin reaction and severe skin rash.high blood pressure.decreased blood flow to the heart and heart attack.More items...
Stivarga Side Effects: Common, Severe, Long Term - Drugs.com
Stivarga Side Effects. Generic name: regorafenib Medically reviewed by Drugs.com. Last updated on Apr 16, 2022. Consumer; Professional; Note: This document contains side effect information about regorafenib. Some of the dosage forms listed on this page may not apply to the brand name Stivarga.. Summary
Side Effects of Stivarga (Regorafenib Tablets), Warnings, Uses - RxList
Gastrointestinal Stromal Tumors. The safety data described below are derived from a randomized (2:1), double-blind, placebo-controlled trial (GRID) in which 132 patients (median age 60 years; 64% men) with previously-treated GIST received STIVARGA as a single agent at a dose of 160 mg daily for the first 3 weeks of each 4 week treatment cycle and 66 patients (median age 61 years; 64% men ...
Stivarga: Uses, Dosage, Side Effects & Warnings - Drugs.com
Generic name: regorafenib [ RE-goe-RAF-e-nib ] Drug classes: Multikinase inhibitors, VEGF/VEGFR inhibitors Medically reviewed by Sanjai Sinha, MD.Last updated on Apr 4, 2022. Warnings; Dosage; Side effects; Interactions; What is Stivarga? Stivarga is a cancer medicine that interferes with the growth and spread of cancer cells in the body.. Stivarga is used to treat colorectal cancer and liver ...
See full prescribing information for complete boxed warning. Severe and ...
3 FULL PRESCRIBING INFORMATION WARNING: HEPATOTOXICITY • Severe and sometimes fatal hepatotoxicity has occurred in clinical trials [see Warnings and Precautions (5.1)]. • Monitor hepatic function prior to and during treatment [see Warnings and Precautions (5.1)]. • Interrupt and then reduce or discontinue STIVARGA for hepatotoxicity as manifested by elevated liver
Regorafenib - Wikipedia
Regorafenib, sold under the brand name Stivarga among others, is an oral multi-kinase inhibitor developed by Bayer which targets angiogenic, stromal and oncogenic receptor tyrosine kinase (RTK). Regorafenib shows anti-angiogenic activity due to its dual targeted VEGFR2-TIE2 tyrosine kinase inhibition.Since 2009 it was studied as a potential treatment option in multiple tumor types.
What is stivarga used for?from stivarga-us.com
STIVARGA is indicated for patients with a type of liver cancer called hepatocellular carcinoma in people who have been previously treated with sorafenib.
What is access services by Bayer?from hcp.nexavar-us.com
Access Services by Bayer ™ is a free support program available to eligible patients who have been prescribed NEXAVAR ® (sorafenib). Access Services by Bayer provides patients with information about their therapy, helps them evaluate their financial assistance options, and offers education and support to health care professionals.
What are the most common laboratory abnormalities observed in the Nexavar study?from hcp.nexavar-us.com
In the DTC study, the most common laboratory abnormalities observed in the NEXAVAR arm versus the placebo arm, respectively, were elevated ALT (59% vs. 24%), elevated AST (54% vs. 15%), and hypocalcemia (36% vs. 11%). The relative increase for the following laboratory abnormalities observed in NEXAVAR-treated DTC patients as compared to placebo-treated patients is similar to that observed in the RCC and HCC studies: lipase, amylase, hypokalemia, hypophosphatemia, neutropenia, lymphopenia, anemia, and thrombocytopenia.
How long after stivarga do you need to use contraception?from hcp.stivarga-us.com
Advise females of reproductive potential and males with female partners of reproductive potential to use effective contraception during treatment with STIVARGA and for 2 months after the final dose.
How long to wait to take Nexavar after surgery?from hcp.nexavar-us.com
Withhold NEXAVAR for at least 10 days prior to elective surgery. Do not administer for at least 2 weeks following major surgery and until adequate wound healing.
What is Nexavar used for?from hcp.nexavar-us.com
NEXAVAR is indicated for the treatment of patients with advanced renal cell carcinoma (RCC). NEXAVAR is indicated for the treatment of patients with locally recurrent or metastatic, progressive, differentiated thyroid carcinoma (DTC) that is refractory to radioactive iodine treatment.
How long after stivarga can you breastfeed?from hcp.stivarga-us.com
are breast-feeding or plan to breast-feed. It is not known if STIVARGA passes into your breast milk. Do not breastfeed during treatment with STIVARGA and for 2 weeks after your final dose of STIVARGA
What is stivarga used for?
STIVARGA is indicated for patients with a type of liver cancer called hepatocellular carcinoma in people who have been previously treated with sorafenib.
How long after stivarga can you breastfeed?
are breast-feeding or plan to breast-feed. It is not known if STIVARGA passes into your breast milk. Do not breastfeed during treatment with STIVARGA and for 2 weeks after your final dose of STIVARGA
Can you die from stiverga?
severe bleeding. STIVARGA can cause bleeding, which can be serious and sometimes lead to death. Tell your healthcare provider if you have any signs of bleeding while taking STIVARGA, including: vomiting blood or if your vomit looks like coffee grounds, pink or brown urine, red or black (looks like tar) stools, coughing up blood or blood clots, menstrual bleeding that is heavier than normal, unusual vaginal bleeding, nose bleeds that happen often, bruising, and lightheadedness
Does stiverga cause diarrhea?
The most common side effects with STIVARGA include pain including stomach-area (abdomen); tiredness, weakness, fatigue; diarrhea (frequent or loose bowel movements); decreased appetite; infection; voice change or hoarseness; increase in certain liver function tests; fever; swelling, pain, and redness of the lining in your mouth, throat, stomach, and bowel (mucositis); and weight loss.
Can you die from taking stivarga?
STIVARGA (regorafenib) can cause liver problems, which can be serious and sometimes lead to death. Your healthcare provider will do blood tests to check your liver function before you start taking STIVARGA and during your treatment with STIVARGA to check for liver problems.
Does stivarga cause infections?
STIVARGA may cause serious side effects, including: Infection. STIVARGA may lead to a higher risk of infections especially of the urinary tract, nose, throat and lung. STIVARGA may lead to a higher risk of fungal infections of the mucous membrane, skin or the body.
How to report Bayer products?
You are encouraged to report side effects or quality complaints of products to the FDA by visiting www.fda.gov/medwatch or calling 1-800-FDA-1088. For Bayer products, you can report these directly to Bayer by clicking here.
How long to wait to give stivarga after surgery?
Therefore, STIVARGA has the potential to adversely affect wound healing. Withhold STIVARGA for at least 2 weeks prior to elective surgery. Do not administer for at least 2 weeks following major surgery and until adequate wound healing.
How long after stivarga can you breastfeed?
Nursing Mothers: Because of the potential for serious adverse reactions in breastfed infants from STIVARGA, do not breastfeed during treatment with STIVARGA and for 2 weeks after the final dose.
How long after stivarga do you need to use contraception?
Advise females of reproductive potential and males with female partners of reproductive potential to use effective contraception during treatment with STIVARGA and for 2 months after the final dose.
Does stivarga cause urinary tract infections?
Infections: STIVARGA caused an increased risk of infections. The overall incidence of infection (Grades 1-5) was higher (32% vs 17%) in 1142 STIVARGA-treated patients as compared to the control arm in randomized placebo-controlled trials. The incidence of grade 3 or greater infections in STIVARGA treated patients was 9%. The most common infections were urinary tract infections (5.7%), nasopharyngitis (4.0%), mucocutaneous and systemic fungal infections (3.3%) and pneumonia (2.6%). Fatal outcomes caused by infection occurred more often in patients treated with STIVARGA (1.0%) as compared to patients receiving placebo (0.3%); the most common fatal infections were respiratory (0.6% vs 0.2%). Withhold STIVARGA for Grade 3 or 4 infections, or worsening infection of any grade. Resume STIVARGA at the same dose following resolution of infection.
Does vicarga cause hemorrhage?
Hemorrhage: STIVARGA caused an increased incidence of hemorrhage. The overall incidence (Grades 1-5) was 18.2% in 1142 patients treated with STIVARGA vs 9.5% with placebo in randomized, placebo-controlled trials. The incidence of grade 3 or greater hemorrhage in patients treated with STIVARGA was 3.0%. The incidence of fatal hemorrhagic events was 0.7%, involving the central nervous system or the respiratory, gastrointestinal, or genitourinary tracts. Permanently discontinue STIVARGA in patients with severe or life-threatening hemorrhage and monitor INR levels more frequently in patients receiving warfarin.
Does stiverga cause hypertension?
Hypertension: Hypertensive crisis occurred in 0.2% in STIVARGA-treated patients and in none of the patients in placebo arm across all randomized, placebo-controlled trials. STIVARGA caused an increased incidence of hypertension (30% vs 8% in mCRC, 59% vs 27% in GIST, and 31% vs 6% in HCC). The onset of hypertension occurred during the first cycle of treatment in most patients who developed hypertension (67% in randomized, placebo controlled trials). Do not initiate STIVARGA until blood pressure is adequately controlled. Monitor blood pressure weekly for the first 6 weeks of treatment and then every cycle, or more frequently, as clinically indicated. Temporarily or permanently withhold STIVARGA for severe or uncontrolled hypertension.
What is access services by Bayer?
Access Services by Bayer is a free support program available to eligible patients who have been prescribed STIVARGA ® (regorafenib). Access Services by Bayer provides patients with information about their therapy, helps them evaluate their financial assistance options, and offers education and support to healthcare professionals.
How to report Bayer products?
You are encouraged to report side effects or quality complaints of products to the FDA by visiting www.fda.gov/medwatch or calling 1-800-FDA-1088. For Bayer products, you can report these directly to Bayer by clicking here.
How long after stivarga do you need to use contraception?
Advise females of reproductive potential and males with female partners of reproductive potential to use effective contraception during treatment with STIVARGA and for 2 months after the final dose.
How long to withhold vicarga?
Withhold STIVARGA for at least 2 weeks prior to elective surgery. Do not administer for at least 2 weeks following major surgery and until adequate wound healing. The safety of resumption of STIVARGA after resolution of wound healing complications has not been established.
Does stivarga cause urinary tract infections?
Infections: STIVARGA caused an increased risk of infections. The overall incidence of infection (Grades 1-5) was higher (32% vs 17%) in 1142 STIVARGA-treated patients as compared to the control arm in randomized placebo-controlled trials. The incidence of grade 3 or greater infections in STIVARGA treated patients was 9%. The most common infections were urinary tract infections (5.7%), nasopharyngitis (4.0%), mucocutaneous and systemic fungal infections (3.3%) and pneumonia (2.6%). Fatal outcomes caused by infection occurred more often in patients treated with STIVARGA (1.0%) as compared to patients receiving placebo (0.3%); the most common fatal infections were respiratory (0.6% vs 0.2%). Withhold STIVARGA for Grade 3 or 4 infections, or worsening infection of any grade. Resume STIVARGA at the same dose following resolution of infection.
Can you stop stivarga?
Interrupt and then reduce or discontinue STIVARGA for hepatotoxicity as manifested by elevated liver function tests or hepatocellular necrosis, depending upon severity and persistence.
Does vicarga cause hemorrhage?
Hemorrhage: STIVARGA caused an increased incidence of hemorrhage. The overall incidence (Grades 1-5) was 18.2% in 1142 patients treated with STIVARGA vs 9.5% with placebo in randomized, placebo-controlled trials. The incidence of grade 3 or greater hemorrhage in patients treated with STIVARGA was 3.0%. The incidence of fatal hemorrhagic events was 0.7%, involving the central nervous system or the respiratory, gastrointestinal, or genitourinary tracts. Permanently discontinue STIVARGA in patients with severe or life-threatening hemorrhage and monitor INR levels more frequently in patients receiving warfarin.
How long after stivarga can you get pregnant?
Females and males should use effective birth control during treatment with STIVARGA and for 2 months after their last dose of STIVARGA. Tell your healthcare provider right away if you or your partner becomes pregnant either while taking STIVARGA or within 2 months after your last dose of STIVARGA
Is a patient a resident of the United States?
The patient is a United States Resident.
Does stiverga cause diarrhea?
The most common side effects with STIVARGA include pain including stomach-area (abdomen); tiredness, weakness, fatigue; diarrhea (frequent or loose bowel movements); decreased appetite; infection; voice change or hoarseness; increase in certain liver function tests; fever; swelling, pain, and redness of the lining in your mouth, throat, stomach, and bowel (mucositis); and weight loss
Can you die from stiverga?
severe bleeding. STIVARGA can cause bleeding, which can be serious and sometimes lead to death. Tell your healthcare provider if you have any signs of bleeding while taking STIVARGA, including: vomiting blood or if your vomit looks like coffee grounds, pink or brown urine, red or black (looks like tar) stools, coughing up blood or blood clots, menstrual bleeding that is heavier than normal, unusual vaginal bleeding, nose bleeds that happen often, bruising, and lightheadedness
Can you die from taking stivarga?
STIVARGA (regorafenib) can cause liver problems, which can be serious and sometimes lead to death. Your healthcare provider will do blood tests to check your liver function before you start taking STIVARGA and during your treatment with STIVARGA to check for liver problems.
Can a patient submit a portion of a copay to a federal or state healthcare program?
Patient agrees not to submit any portion toward the product dispensed pursuant to this co-pay program to a federal or state healthcare program for purposes of counting it toward the patient's out-of-pocket expenses (such as Medicaid)
Does Bayer cover administrative costs?
The program does not cover costs associated with a patient visit including prescriber, staff, or administrative charges associated with administering the applicable Bayer product
What is Bayer US Patient Assistance Foundation?from bayer.com
The Bayer US Patient Assistance Foundation is a charitable organization that helps eligible patients get their Bayer prescription medication at no cost.
What is Mirena offer?from drugs.com
Mirena offers may be in the form of a printable coupon, rebate, savings card, trial offer, or free samples. Some offers may be printed right from a website, others require registration, completing a questionnaire, or obtaining a sample from the doctor's office.
How much does Stivarga cost?
The cost for Stivarga oral tablet 40 mg is around $19,581 for a supply of 84, depending on the pharmacy you visit. Prices are for cash paying customers only and are not valid with insurance plans.
What is stivarga used for?
Stivarga (regorafenib) is a member of the multikinase inhibitors drug class and is commonly used for Colorectal Cancer, Gastrointestinal Stromal Tumor, and Hepatocellular Carcinoma.
What is stivarga used for?
STIVARGA is indicated for patients with a type of liver cancer called hepatocellular carcinoma in people who have been previously treated with sorafenib.
How long after stivarga can you breastfeed?
are breast-feeding or plan to breast-feed. It is not known if STIVARGA passes into your breast milk. Do not breastfeed during treatment with STIVARGA and for 2 weeks after your final dose of STIVARGA
Can you die from stiverga?
severe bleeding. STIVARGA can cause bleeding, which can be serious and sometimes lead to death. Tell your healthcare provider if you have any signs of bleeding while taking STIVARGA, including: vomiting blood or if your vomit looks like coffee grounds, pink or brown urine, red or black (looks like tar) stools, coughing up blood or blood clots, menstrual bleeding that is heavier than normal, unusual vaginal bleeding, nose bleeds that happen often, bruising, and lightheadedness
Does stiverga cause diarrhea?
The most common side effects with STIVARGA include pain including stomach-area (abdomen); tiredness, weakness, fatigue; diarrhea (frequent or loose bowel movements); decreased appetite; infection; voice change or hoarseness; increase in certain liver function tests; fever; swelling, pain, and redness of the lining in your mouth, throat, stomach, and bowel (mucositis); and weight loss.
Can you die from taking stivarga?
STIVARGA (regorafenib) can cause liver problems, which can be serious and sometimes lead to death. Your healthcare provider will do blood tests to check your liver function before you start taking STIVARGA and during your treatment with STIVARGA to check for liver problems.
Does stivarga cause infections?
STIVARGA may cause serious side effects, including: Infection. STIVARGA may lead to a higher risk of infections especially of the urinary tract, nose, throat and lung. STIVARGA may lead to a higher risk of fungal infections of the mucous membrane, skin or the body.
