
When will Celgene approve OTEZLA?
What is OTezla approved for?
How effective is otezla?
How many patients are prescribed otezla?
How much weight loss is associated with Behçet's disease?
Is the OTEZLA study placebo controlled?
Is OTezla approved for psoriatic arthritis?
See 2 more
About this website
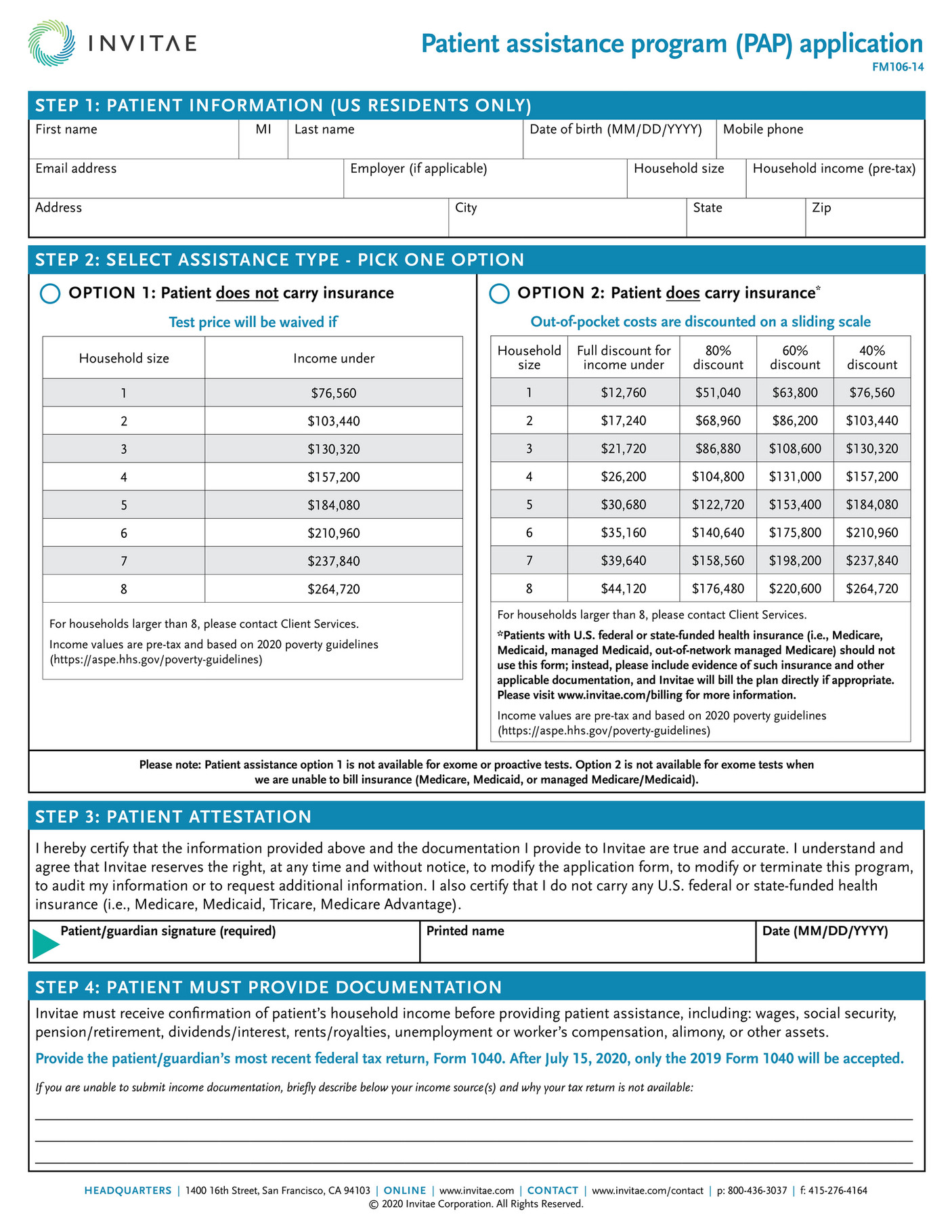
What is the Otezla bridge program?
The program provides up to a Maximum Program Benefit of assistance to reduce a patient's out-of-pocket prescription costs that Amgen will provide per patient for each calendar year, which must be applied to the Otezla patient's out-of-pocket costs (co-pay, deductible, or co-insurance).
How do you get prescribed Otezla starter pack?
START NOW3 SIMPLE STEPS TO GET STARTED.Provide an Otezla Starter Pack so patients can start taking Otezla right away. ... Send prescriptions to the specialty pharmacy (SP) of your choice. ... Initiate a prior authorization (PA) process. ... Send prescriptions to the specialty pharmacy (SP) of your choice.More items...
How many days is Otezla starter pack?
When you first start Otezla, your doctor will give you a 14-day Starter Pack. All of the instructions are printed on the pack. During the first 5 days you gradually increase your dose until you reach the 30 mg twice a day. People with severe kidney disease will follow a different schedule for increasing their dose.
What company makes Otezla?
Otezla® is a registered trademark of Celgene Corporation . Please see Full Prescribing Information for ENBREL.
Is there a generic drug for Otezla?
Otezla is a specialty drug used to treat autoimmune conditions such as psoriatic arthritis and plaque psoriasis. Otezla is one of the most expensive drugs commonly prescribed in the U.S., and there is no generic version on the market.
Is Otezla hard to get?
Getting Otezla shouldn't be difficult. If you don't hear from your specialty pharmacy within a week after your doctor submitted your prescription, be sure to give Otezla SupportPlus a call at 1-844-4OTEZLA (1-844-468-3952). *The Otezla Co-Pay Card is for eligible commercially insured patients.
How long do you stay on Otezla?
For some people, Otezla can prevent symptoms of psoriasis or psoriatic arthritis without causing any problematic side effects. In this case, the drug may be continued long-term to keep the condition under control. Long-term studies, ranging from 3 to 5 years, have shown that Otezla is safe for prolonged use.
What medications make Otezla less effective?
Other medications can affect the removal of apremilast from your body, which may affect how apremilast works. Examples include certain drugs used to treat seizures (such as carbamazepine, phenobarbital, phenytoin, primidone), rifamycins (such as rifampin, rifabutin), among others.
When do Otezla side effects start?
This typically happens within about 2 weeks after starting the drug. Also keep in mind that side effects can depend on factors such as your age, other health conditions you have, or other medications you're taking. If you have side effects of Otezla that become severe or don't go away, talk with your doctor.
Is Otezla a Tier 4 drug?
What drug tier is Otezla typically on? Medicare prescription drug plans typically list Otezla on Tier 5 of their formulary.
Does Otezla weaken immune system?
Yes, Otezla is an immunosuppressant. This means the drug lowers (suppresses) the activity of your immune system. Specifically, Otezla works by reducing inflammation that's caused by an overactive immune system.
What is the new pill for psoriasis?
FDA: "FDA approves new psoriasis drug Taltz,” "FDA approves Amjevita, a biosimilar to Humira."
How many pills come in the Otezla starter pack?
Otezla Starter Pack (14-day or 28-day) 1. If the individual requires additional induction dosing, as verified by the absence of claims for Otezla in the past 130 days, approve a one-time override for one 14-day Starter Pack (27 tablets) or one 28-day Starter Pack (55 tablets).
When is Otezla prescribed?
Otezla® (apremilast) is a prescription medicine used to treat adult patients with: Plaque psoriasis for whom phototherapy or systemic therapy is appropriate. Active psoriatic arthritis. Oral ulcers associated with Behçet's Disease.
Does Otezla go to a specialty pharmacy?
Otezla is delivered by a specialty pharmacy. What does that mean? Unlike a retail pharmacy, where you go to pick up your medication, specialty pharmacies deliver your Otezla straight to your door.
Does Otezla work psoriasis?
Otezla is the first and only pill for mild to moderate scalp psoriasis, with nearly 3 times as many people achieving clearer skin—44% of people taking Otezla saw improvement after 4 months vs 17% of those taking a placebo.
When will Celgene approve OTEZLA?
Celgene anticipates a regulatory decision for OTEZLA in oral ulcers associated with Behçet’s Disease from the Pharmaceuticals and Medical Devices Agency in Japan in the second half of 2019. The Company also submitted a Type II Variation to the Marketing Authorization Application earlier this year seeking approval in the European Union.
What is OTezla approved for?
FDA Approves OTEZLA® (apremilast) for the Treatment of Oral Ulcers Associated with Behçet’s Disease. With this third indication in the U.S., OTEZLA is the first and only treatment approved for oral ulcers associated with Behçet’s Disease.
How effective is otezla?
The FDA approval was based on efficacy and safety results from the randomized, placebo-controlled, double-blind Phase 3 RELIEF™ study evaluating OTEZLA in 207 adult patients with Behçet’s Disease with active oral ulcers who were previously treated with at least one nonbiologic medication and were candidates for systemic therapy. Results showed OTEZLA 30 mg BID resulted in a 42.7 point reduction from baseline in the pain of oral ulcers as measured by the visual analog scale (VAS) at week 12, compared with an 18.7 point reduction with placebo. The proportion of patients achieving an oral ulcer complete response (oral ulcer-free) at week 12 was 52.9% in the OTEZLA arm and 22.3% in the placebo arm. The proportion of patients achieving oral ulcer complete response by week 6 and who remained oral ulcer-free for at least six additional weeks during the 12-week treatment phase was 29.8% in the OTEZLA arm and 4.9% in the placebo arm. The daily average number of oral ulcers during the 12-week treatment phase was 1.5 in the OTEZLA arm and 2.6 in the placebo arm (based on oral ulcer counts measured at baseline and at weeks 1, 2, 4, 6, 8, 10 and 12).
How many patients are prescribed otezla?
Since its initial FDA approval in 2014, OTEZLA has been prescribed to more than 250,000 patients with moderate to severe plaque psoriasis or active psoriatic arthritis in the U.S. 4. OTEZLA is available in the U.S. and is dispensed through a comprehensive network of specialty pharmacies.
How much weight loss is associated with Behçet's disease?
Behçet’s Disease: Body weight loss of >5% was reported in 4.9% (5/103) of patients taking Otezla and in 3.9% (4/102) of patients taking placebo.
Is the OTEZLA study placebo controlled?
The RELIEF™ study is a Phase 3 randomized , placebo-controlled, double-blind study evaluating OTEZLA 30 mg BID in 207 adult patients with Behçet’s Disease with active oral ulcers who were previously treated with at least one nonbiologic medication and were candidates for systemic therapy. This 64-week study was conducted at 53 sites across 10 countries.
Is OTezla approved for psoriatic arthritis?
OTEZLA is now approved for three indications in the U.S., including the treatment of patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy, adult patients with active psoriatic arthritis and adult patients with oral ulcers associated with Behçet’s Disease.
What is otezla used for?
Otezla®(apremilast) is indicated for the treatment of adult patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy. Otezla is indicated for the treatment of adult patients with active psoriatic arthritis.
What are the warnings and precautions for Otezla?
Warnings and Precautions. Diarrhea, Nausea, and Vomiting: Cases of severe diarrhea, nausea, and vomiting were associated with the use of Otezla. Most events occurred within the first few weeks of treatment.
Does apremilast affect milk production?
Lactation: There are no data on the presence of apremilast or its metabolites in human milk, the effects of apremilast on the breastfed infant, or the effects of the drug on milk production . The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Otezla and any potential adverse effects on the breastfed child from Otezla or from the underlying maternal condition
Does otezla cause depression?
Psoriatic Arthritis: Treatment with Otezla is associated with an increase in depression. During clinical trials, 1.0% (10/998) reported depression or depressed mood compared to 0.8% (4/495) treated with placebo.
Is otezla safe for pregnant women?
Pregnancy : Otezla has not been studied in pregnant women. Advise pregnant women of the potential risk of fetal loss. Consider pregnancy planning and prevention for females of reproductive potential. There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to Otezla during pregnancy.
Is otezla contraindicated for Behçet's disease?
Otezla is indicated for the treatment of adult patients with oral ulcers associated with Behçet’s Disease. IMPORTANT SAFETY INFORMATION. Contraindications. Otezla®(apremilast) is contraindicated in patients with a known hypersensitivity to apremilast or to any of the excipients in the formulation.
IMPORTANT SAFETY INFORMATION
Diarrhea, Nausea, and Vomiting: Cases of severe diarrhea, nausea, and vomiting were associated with the use of Otezla. Most events occurred within the first few weeks of treatment. In some cases patients were hospitalized.
INDICATIONS
Otezla ® (apremilast) is indicated for the treatment of adult patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy.
When will Celgene approve OTEZLA?
Celgene anticipates a regulatory decision for OTEZLA in oral ulcers associated with Behçet’s Disease from the Pharmaceuticals and Medical Devices Agency in Japan in the second half of 2019. The Company also submitted a Type II Variation to the Marketing Authorization Application earlier this year seeking approval in the European Union.
What is OTezla approved for?
FDA Approves OTEZLA® (apremilast) for the Treatment of Oral Ulcers Associated with Behçet’s Disease. With this third indication in the U.S., OTEZLA is the first and only treatment approved for oral ulcers associated with Behçet’s Disease.
How effective is otezla?
The FDA approval was based on efficacy and safety results from the randomized, placebo-controlled, double-blind Phase 3 RELIEF™ study evaluating OTEZLA in 207 adult patients with Behçet’s Disease with active oral ulcers who were previously treated with at least one nonbiologic medication and were candidates for systemic therapy. Results showed OTEZLA 30 mg BID resulted in a 42.7 point reduction from baseline in the pain of oral ulcers as measured by the visual analog scale (VAS) at week 12, compared with an 18.7 point reduction with placebo. The proportion of patients achieving an oral ulcer complete response (oral ulcer-free) at week 12 was 52.9% in the OTEZLA arm and 22.3% in the placebo arm. The proportion of patients achieving oral ulcer complete response by week 6 and who remained oral ulcer-free for at least six additional weeks during the 12-week treatment phase was 29.8% in the OTEZLA arm and 4.9% in the placebo arm. The daily average number of oral ulcers during the 12-week treatment phase was 1.5 in the OTEZLA arm and 2.6 in the placebo arm (based on oral ulcer counts measured at baseline and at weeks 1, 2, 4, 6, 8, 10 and 12).
How many patients are prescribed otezla?
Since its initial FDA approval in 2014, OTEZLA has been prescribed to more than 250,000 patients with moderate to severe plaque psoriasis or active psoriatic arthritis in the U.S. 4. OTEZLA is available in the U.S. and is dispensed through a comprehensive network of specialty pharmacies.
How much weight loss is associated with Behçet's disease?
Behçet’s Disease: Body weight loss of >5% was reported in 4.9% (5/103) of patients taking Otezla and in 3.9% (4/102) of patients taking placebo.
Is the OTEZLA study placebo controlled?
The RELIEF™ study is a Phase 3 randomized , placebo-controlled, double-blind study evaluating OTEZLA 30 mg BID in 207 adult patients with Behçet’s Disease with active oral ulcers who were previously treated with at least one nonbiologic medication and were candidates for systemic therapy. This 64-week study was conducted at 53 sites across 10 countries.
Is OTezla approved for psoriatic arthritis?
OTEZLA is now approved for three indications in the U.S., including the treatment of patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy, adult patients with active psoriatic arthritis and adult patients with oral ulcers associated with Behçet’s Disease.
