Is Lenvatinib covered by Medicare?
Yes. 100% of Medicare prescription drug plans cover this drug.
Who is the manufacturer of Lenvima?
EISAI AND MERCK & CO., INC., KENILWORTH, N.J., U.S.A. ANNOUNCE FDA APPROVAL OF LENVIMA® (LENVATINIB) CAPSULES FOR FIRST-LINE TREATMENT OF UNRESECTABLE HEPATOCELLULAR CARCINOMA (HCC)
Is there a generic for Lenvima?
Lenvatinib is the generic for the trade name drug Lenvima™. In some cases, health care professionals may use the trade name Lenvima™ when referring to the generic drug name lenvatinib. Drug type: Lenvatinib is a targeted therapy.
How effective is Lenvima?
Lenvima has been shown to be at least as effective as the cancer medicine sorafenib at prolonging the time patients lived in one main study. The study involved 954 patients with hepatocellular carcinoma who had not previously been treated for their cancer and whose cancer could not be removed by surgery.
What is the price of Lenvima?
The cost for Lenvima oral capsule (14 mg daily-dose) is around $22,452 for a supply of 60, depending on the pharmacy you visit. Prices are for cash paying customers only and are not valid with insurance plans.
Does Lenvima shrink tumors?
A median is the middle number in a list of numbers arranged from lowest to highest. Almost 3.5 times as many patients treated with LENVIMA (41%) had their tumors shrink compared with patients treated with sorafenib (12%).
How long can you take Lenvima?
You will take Lenvima (lenvatinib) until your body no longer responds to the medication or the side effects become too severe for you to tolerate. In safety studies conducted by the manufacturer, most patients took Lenvima for 6 to 16 months for the treatment of various types of cancer.
Is Lenvima chemotherapy?
It is an antineoplastic (anti-cancer) drug that belongs to a class called tyrosine kinase inhibitors (TKI) of vascular endothelial growth factor (VEGF) receptors. TKIs work by inhibiting the enzyme tyrosine kinase, thus blocking cell growth and division. Lenvima is a type of chemotherapy.
When does Lenvima go off patent?
Lenvima was eligible for patent challenges on February 13, 2019. By analyzing the patents and regulatory protections it appears that the earliest date for generic entry will be August 26, 2035.
Does Lenvima cause fatigue?
The most common side effects of LENVIMA in people treated for thyroid cancer include tiredness; joint and muscle pain; decreased appetite; weight loss; nausea; mouth sores; headache; vomiting; rash, redness, itching, or peeling of your skin on your hands and feet; stomach (abdomen) pain; and hoarseness.
Does Lenvima cause hair loss?
All drugs may cause side effects. However, many people have no side effects or only have minor side effects. Call your doctor or get medical help if any of these side effects or any other side effects bother you or do not go away: Hair loss.
Is Lenvima an immunotherapy?
For example, lenvatinib is used in combination with pembrolizumab (Keytruda), an immunotherapy, for the treatment of endometrial cancer.
How long has Lenvima been on the market?
In May 2016, LENVIMA was approved in the U.S. in combination with everolimus, for patients with advanced renal cell carcinoma (RCC) following one prior anti-angiogenic therapy. Under the collaboration, Eisai and Merck initiated co-commercialization activities for LENVIMA in the U.S. in June 2018.
Is Lenvima chemotherapy?
It is an antineoplastic (anti-cancer) drug that belongs to a class called tyrosine kinase inhibitors (TKI) of vascular endothelial growth factor (VEGF) receptors. TKIs work by inhibiting the enzyme tyrosine kinase, thus blocking cell growth and division. Lenvima is a type of chemotherapy.
When was Lenvima approved by the FDA?
On August 10, 2021, FDA approved the combination of lenvatinib (brand name Lenvima) plus pembrolizumab (brand name Keytruda) for first-line treatment of adult patients with advanced renal cell carcinoma.
What class of drug is Lenvima?
This medication is used to treat cancer. Lenvatinib belongs to a class of drugs known as tyrosine kinase inhibitors. It works by slowing or stopping the growth of cancer cells.
How long to withhold leniva?
Withhold LENVIMA for at least 1 week prior to elective surgery. Do not administer for at least 2 weeks following major surgery and until adequate wound healing. The safety of resumption of LENVIMA after resolution of wound healing complications has not been established. Osteonecrosis of the Jaw (ONJ).
How long after leniva can you stop breastfeeding?
Because of the potential for serious adverse reactions in breastfed infants, advise women to discontinue breastfeeding during treatment and for at least 1 week after the last dose. LENVIMA may impair fertility in males and females of reproductive potential.#N#No dose adjustment is recommended for patients with mild (CLcr 60-89 mL/min) or moderate (CLcr 30-59 mL/min) renal impairment. LENVIMA concentrations may increase in patients with DTC or RCC and severe (CLcr 15-29 mL/min) renal impairment. Reduce the dose for patients with RCC or DTC and severe renal impairment. There is no recommended dose for patients with HCC and severe renal impairment. LENVIMA has not been studied in patients with end-stage renal disease.#N#No dose adjustment is recommended for patients with HCC and mild hepatic impairment (Child-Pugh A). There is no recommended dose for patients with HCC with moderate (Child-Pugh B) or severe (Child-Pugh C) hepatic impairment.#N#No dose adjustment is recommended for patients with DTC or RCC and mild or moderate hepatic impairment. LENVIMA concentrations may increase in patients with DTC or RCC and severe hepatic impairment. Reduce the dose for patients with DTC or RCC and severe hepatic impairment.
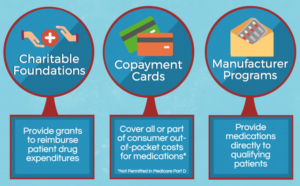
Does Eisai have a patient assistance program?
Eisai has established the Patient Assistance Program for patients who need help paying for LENVIMA ®. This program provides LENVIMA ® at no cost to uninsured and financially burdened patients who meet the program eligibility criteria.
Advocacy organizations
The below advocacy groups offer support and advice for cancer patients and their caregivers.
Receiving your prescription
There are multiple ways to receive your LENVIMA prescription. It could be through a Specialty Pharmacy, your doctor’s office/clinic, or a hospital pharmacy.
What is the Eisai program?
The Eisai Assistance Program is your resource for information about coverage of LENVIMA and available financial assistance options. It will help you: Understand if your medicine is covered by insurance. Determine eligibility for assistance if you cannot afford your medication. Visit the Eisai Assistance Program.
How to contact Eisai?
Contact the Eisai Assistance Program directly at 1-866-613-4724. The Eisai Assistance Program is your resource for information about coverage of LENVIMA and available financial assistance options.
How long to wait to administer lenvima after surgery?
Impaired Wound Healing. Impaired wound healing has been reported in patients who received LENVIMA. Withhold LENVIMA for at least 1 week prior to elective surgery. Do not administer for at least 2 weeks following major surgery and until adequate wound healing. The safety of resumption of LENVIMA after resolution of wound healing complications has not been established.
What is the esai number?
Please call the Eisai Assistance Program at 1-866-61-EISAI (1-866-613-4724) for more information.
What is RPLS in MRI?
Reversible Posterior Leukoencephalopathy Syndrome (RPLS). Across clinical studies of 1823 patients who received LENVIMA as a single agent, RPLS occurred in 0.3%. Confirm diagnosis of RPLS with MRI. Withhold and resume at reduced dose upon recovery or permanently discontinue depending on severity and persistence of neurologic symptoms.
How long after leniva can you breastfeed?
Because of the potential for serious adverse reactions in breastfed infants, advise women to discontinue breastfeeding during treatment and for at least 1 week after the last dose. LENVIMA may impair fertility in males and females of reproductive potential.
What is lenvigam used for?
LENVIMA is indicated for the first-line treatment of patients with unresectable hepatocellular carcinoma (HCC).
Does leniva cause diarrhea?
Diarrhea. Of the 737 LENVIMA-treated patients in DTC and HCC, diarrhea occurred in 49% (6% grade 3). In RCC, diarrhea occurred in 81% of LENVIMA + everolimus–treated patients (19% grade 3). Diarrhea was the most frequent cause of dose interruption/reduction, and diarrhea recurred despite dose reduction. Promptly initiate management of diarrhea. Withhold and resume at reduced dose upon recovery or permanently discontinue based on severity.
Does Eisai pay for claims?
Eisai cannot guarantee payment of any claim. Coding, coverage, and reimbursement may vary significantly by payer, plan, patient, and setting of care. Actual coverage and reimbursement decisions are made by individual payers following the receipt of claims.
How long to wait to administer lenvima after surgery?
Impaired Wound Healing. Impaired wound healing has been reported in patients who received LENVIMA. Withhold LENVIMA for at least 1 week prior to elective surgery. Do not administer for at least 2 weeks following major surgery and until adequate wound healing. The safety of resumption of LENVIMA after resolution of wound healing complications has not been established.
What is the grade of hypertension in leniva?
Hypertension. In DTC (differentiated thyroid cancer), hypertension occurred in 73% of patients on LENVIMA (44% grade 3-4). In RCC (renal cell carcinoma), hypertension occurred in 42% of patients on LENVIMA + everolimus (13% grade 3). Systolic blood pressure ≥160 mmHg occurred in 29% of patients, and 21% had diastolic blood pressure ≥100 mmHg. In HCC (hepatocellular carcinoma), hypertension occurred in 45% of LENVIMA-treated patients (24% grade 3). Grade 4 hypertension was not reported in HCC.
What is RPLS in MRI?
Reversible Posterior Leukoencephalopathy Syndrome (RPLS). Across clinical studies of 1823 patients who received LENVIMA as a single agent, RPLS occurred in 0.3%. Confirm diagnosis of RPLS with MRI. Withhold and resume at reduced dose upon recovery or permanently discontinue depending on severity and persistence of neurologic symptoms.
What is the esai number?
Please call the Eisai Assistance Program at 1-866-61-EISAI (1-866-613-4724) for more information.
How long after leniva can you breastfeed?
Because of the potential for serious adverse reactions in breastfed infants, advise women to discontinue breastfeeding during treatment and for at least 1 week after the last dose. LENVIMA may impair fertility in males and females of reproductive potential.
What is lenvigam used for?
LENVIMA is indicated in combination with everolimus, for the treatment of adult patients with advanced renal cell carcinoma (RCC) following one prior anti-angiogenic therapy.
Does leniva cause diarrhea?
Diarrhea. Of the 737 LENVIMA-treated patients in DTC and HCC, diarrhea occurred in 49% (6% grade 3). In RCC, diarrhea occurred in 81% of LENVIMA + everolimus–treated patients (19% grade 3). Diarrhea was the most frequent cause of dose interruption/reduction, and diarrhea recurred despite dose reduction. Promptly initiate management of diarrhea. Withhold and resume at reduced dose upon recovery or permanently discontinue based on severity.

Warnings and Precautions
- Hypertension.In DTC, hypertension occurred in 73% of patients on LENVIMA (44% grade 3-4). In RCC, hypertension occurred in 42% of patients on LENVIMA + everolimus (13% grade 3). Systolic blood pressure ≥160 mmHg occurred in 29% of patients, and 21% had diastolic blood pressure ≥100 mmHg. In HCC, hypertension occurred in 45% of LENVIMA-treated patients (24% grade 3). …
Adverse Reactions
- In DTC, the most common adverse reactions (≥30%) observed in LENVIMA-treated patients were hypertension (73%), fatigue (67%), diarrhea (67%), arthralgia/myalgia (62%), decreased appetite (54%), decreased weight (51%), nausea (47%), stomatitis (41%), headache (38%), vomiting (36%), proteinuria (34%), palmar-plantar erythrodysesthesia syndrome (32%), abdominal pain (31%), an…
Use in Specific Populations
- Because of the potential for serious adverse reactions in breastfed infants, advise women to discontinue breastfeeding during treatment and for at least 1 week after the last dose. LENVIMA may impair fertility in males and females of reproductive potential. No dose adjustment is recommended for patients with mild (CLcr 60-89 mL/min) or moderate (CL...