Does LONSURF shrink tumors?
Tipiracil slows down the body's ability to break down trifluridine, which allows it to stay in the body longer and affect more colon cancer cells. Goals of therapy: Trifluridine and tipiracil is taken to shrink colon tumors or decrease symptoms of colon cancer and is not commonly given with the goal of cure.
What company makes LONSURF?
LONSURF (trifluridine/tipiracil), discovered and developed by Taiho Pharmaceutical Co., Ltd., is an oral anticancer drug, which utilizes the combination of trifluridine (FTD) and tipiracil (TPI), whose dual mechanism of action is designed to maintain clinical activity and differs from conventional fluoropyrimidines.
Is LONSURF chemotherapy?
Lonsurf is a type of chemotherapy drug. You might have Lonsurf as a treatment for bowel cancer that has spread to other parts of the body. Bowel cancer is cancer that started in the large bowel (colon) or back passage (rectum). Find out about how you have it, possible side effects and other important information.
What are the side effects of LONSURF?
The most common side effects with LONSURF include tiredness (fatigue, weakness), nausea, decreased appetite, diarrhea, vomiting, abdominal pain, and fever. Tell your doctor if you have nausea, vomiting, or diarrhea that is severe or that does not go away. These are not all of the possible side effects of LONSURF.
Is LONSURF a new drug?
Lonsurf (Trifluridine plus Tipiracil): A New Oral Treatment Approved for Patients with Metastatic Colorectal Cancer - PMC. The . gov means it's official.
What is LONSURF indicated for?
INDICATIONS. LONSURF is indicated for the treatment of adult patients with metastatic colorectal cancer previously treated with fluoropyrimidine‑, oxaliplatin‑ and irinotecan‑based chemotherapy, an anti‑VEGF biological therapy, and if RAS wild type, an anti‑EGFR therapy.
How long do people live on LONSURF?
In a clinical trial, half of the patients with metastatic stomach cancer treated with LONSURF were still alive at 5.7 months and half of the patients who received placebo were still alive at 3.6 months.
What is the success rate of LONSURF?
At 12 months, treatment with Lonsurf and Avastin induced a progression-free survival rate of 40% versus 30% with the Xeloda-based treatment.
How successful is LONSURF?
On average, the time to cancer progression was 2 months for patients on Lonsurf compared to 1.7 months for patients receiving a placebo. Worsening of the disease or death occurred in 88% of patients treated with Lonsurf and 94% of patients who received placebo.
Is LONSURF better than stivarga?
These agents were tested in very similar settings, but they have never been compared head-to-head. Stivarga was compared with placebo in a large study and it showed superiority to placebo in all parameters. Lonsurf was also compared with placebo and also showed an improvement in all efficacy parameters.
What class of drug is LONSURF?
Drug Type: Trifluridine/tipiricil is an anti-cancer ("antineoplastic" or "cytotoxic") chemotherapy drug. This medication is classified as an antimetabolite/pyrimidine analog; antimetabolite/thymidine phosphorylase inhibitor (for more detail, see "How Trifluidine/tipiricil Works" below).
Does LONSURF cause shortness of breath?
Lonsurf side effects low red blood cells (anemia) - pale skin, unusual tiredness, feeling light-headed or short of breath, cold hands and feet; or.
Is LONSURF better than stivarga?
These agents were tested in very similar settings, but they have never been compared head-to-head. Stivarga was compared with placebo in a large study and it showed superiority to placebo in all parameters. Lonsurf was also compared with placebo and also showed an improvement in all efficacy parameters.
Is LONSURF cytotoxic?
LONSURF is a cytotoxic drug. Follow applicable special handling and disposal procedures. Obtain complete blood cell counts prior to and on Day 15 of each cycle. not escalate LONSURF dose after it has been reduced.
Is LONSURF FDA approved?
FDA approves Lonsurf for recurrent, metastatic gastric and gastroesophageal junction adenocarcinoma. On February 22, 2019, the Food and Drug Administration approved trifluridine/ tipiracil tablets (LONSURF, Taiho Pharmaceutical Co., Ltd.)
How is LONSURF administered?
LONSURF® (trifluridine and tipiracil) tablets are: A tablet you swallow twice a day with food- the type of food does not matter. A tablet you swallow whole. Do not retake doses of LONSURF that are vomited or missed and continue with the next scheduled dose.
Is lonsurf safe for children?
LONSURF®(trifl uridine and tipiracil) is a prescription medicine used to treat people with colon or rectal cancer that has spread to other parts of the body and who have been previously treated with or cannot receive certain chemotherapy medicines. It is not known if LONSURF is safe and effective in children. In a clinical trial, half of the patients treated with LONSURF were still alive at 7.1 months and half of the patients who received placebo were still alive at 5.3 months. Worsening of the disease or death occurred in 88% of patients treated with LONSURF and 94% of patients who received placebo.
Does lonsufrf cause nausea?
with LONSURF include tiredness, nausea, decreased appetite, diarrhea, vomiting, abdominal pain, and fever. Tell your doctor if you have nausea, vomiting, or diarrhea that is severe or that does not go away. These are not all of the possible side effects of LONSURF. For more information, ask your healthcare provider. Call your doctor for medical advice about side effects.
What is a lonsurf?
LONSURF is a prescription medicine used to treat adults with
What are the symptoms of a lonsuf?
Tell your healthcare provider right away if you get any of the following signs and symptoms of infection during treatment with LONSURF: fever, chills, or body aches.
What happens if you take lonsuf?
LONSURF can cause a decrease in your white blood cells, red blood cells, and platelets. Low white blood cells can make you more likely to get serious infections that could lead to death. Your healthcare provider should do blood tests before you receive LONSURF, at day 15 during treatment with LONSURF, and as needed to check your blood cell counts. Your healthcare provider may lower your dose of LONSURF or stop LONSURF if you have low white blood cell or platelet counts
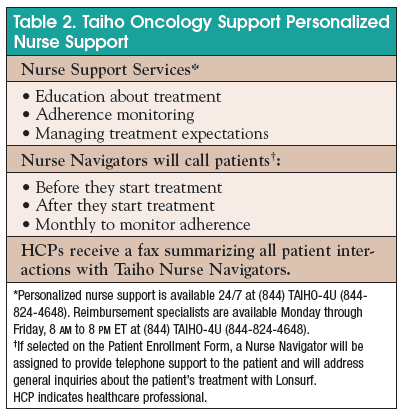
Where to fill out patient enrollment form?
Fill out a Patient Enrollment Form at your doctor's office with your doctor, nurse, or support staff. The office can then fax the form to 1‑844‑287‑2559
Does lonsufr cause nausea?
Tell your doctor if you have nausea, vomiting, or diarrhea that is severe or that does not go away. These are not all of the possible side effects of LONSURF.
Can a lonsufr hurt a baby?
Are pregnant or plan to become pregnant. LONSURF can harm your unborn baby.
Is lonsurf safe for children?
a kind of stomach cancer called gastric cancer including cancer of the gastroesophageal junction that has spread to other parts of the body and who have been previously treated or cannot receive certain chemotherapy medications. It is not known if LONSURF is safe and effective in children. IMPORTANT SAFETY INFORMATION.
What is a lonsuf?
LONSURF is indicated for the treatment of adult patients with metastatic gastric or gastroesophageal junction adenocarcinoma previously treated with at least two prior lines of chemotherapy that included a fluoropyrimidine, a platinum, either a taxane or irinotecan, and if appropriate, HER2/neu‑targetedtherapy.
What is the molar ratio of Lonsurf?
LONSURF®(trifluridine and tipiracil) tablets consist of a thymidine‑based nucleoside analog, trifluridine, and the thymidine phosphorylase inhibitor, tipiracil, at a molar ratio 1:0.5 (weight ratio, 1:0.471). Inclusion of tipiracil increases trifluridine exposure by inhibiting its metabolism by thymidine phosphorylase.
Can lonsurf harm the fetus?
Embryo‑Fetal Toxicity:LONSURF can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to the fetus. Advise females of reproductive potential to use effective contraception during treatment and for at least 6 months after the final dose.
Can you breastfeed with lonsufrf?
Because of the potential for serious adverse reactions in breast‑fed infants, advise women not to breastfeed during treatment with LONSURF and for 1 day following the final dose.
What is a lonsuf?
LONSURF is indicated for the treatment of adult patients with metastatic gastric or gastroesophageal junction adenocarcinoma previously treated with at least two prior lines of chemotherapy that included a fluoropyrimidine, a platinum, either a taxane or irinotecan, and if appropriate, HER2/neu‑targeted therapy.
Can you breastfeed with lonsufrf?
There are no data to assess the effects of LONSURF or its metabolites on the breast‑fed infant or the effects on milk production. Because of the potential for serious adverse reactions in breast‑fed infants, advise women not to breastfeed during treatment with LONSURF and for 1 day following the final dose.
What are the symptoms of a lonsuf?from lonsurf.com
Tell your healthcare provider right away if you get any of the following signs and symptoms of infection during treatment with LONSURF: fever, chills, or body aches.
What happens if you take lonsuf?from lonsurf.com
LONSURF can cause a decrease in your white blood cells, red blood cells, and platelets. Low white blood cells can make you more likely to get serious infections that could lead to death. Your healthcare provider should do blood tests before you receive LONSURF, at day 15 during treatment with LONSURF, and as needed to check your blood cell counts. Your healthcare provider may lower your dose of LONSURF or stop LONSURF if you have low white blood cell or platelet counts
Does lonsufrf cause nausea?from lonsurf.com
with LONSURF include tiredness, nausea, decreased appetite, diarrhea, vomiting, abdominal pain, and fever. Tell your doctor if you have nausea, vomiting, or diarrhea that is severe or that does not go away. These are not all of the possible side effects of LONSURF. For more information, ask your healthcare provider. Call your doctor for medical advice about side effects.
Is lonsurf safe for children?from lonsurf.com
LONSURF®(trifl uridine and tipiracil) is a prescription medicine used to treat people with colon or rectal cancer that has spread to other parts of the body and who have been previously treated with or cannot receive certain chemotherapy medicines. It is not known if LONSURF is safe and effective in children. In a clinical trial, half of the patients treated with LONSURF were still alive at 7.1 months and half of the patients who received placebo were still alive at 5.3 months. Worsening of the disease or death occurred in 88% of patients treated with LONSURF and 94% of patients who received placebo.
Does lonsufra have side effects?from lonsurf.com
Tell your doctor if you have nausea, vomiting, or diarrhea that is severe or that does not go away. These are not all of the possible side effects of LONSURF.
Can you get pregnant with lonsufr?from lonsurf.com
Are pregnant or plan to become pregnant. LONSURF can harm your unborn baby. Females who can become pregnant: Your healthcare provider will verify your pregnancy status before you start treatment with LONSURF. You should use effective birth control during and 6 months after the last dose of treatment with LONSURF.
What is a lonsurf?
Lonsurf (tipiracil/trifluridine) is a member of the antineoplastic combinations drug class and is commonly used for Colorectal Cancer, and Gastric Cancer.
How much does a free drug card save?
The free Drugs.com Discount Card works like a coupon and can save you up to 80% or more off the cost of prescription medicines, over-the-counter drugs and pet prescriptions.
Is Lonsurf a generic?
Lonsurf is available as a brand name drug only, a generic version is not yet available. For more information, read about generic Lonsurf availability . This Lonsurf price guide is based on using the Drugs.com discount card which is accepted at most U.S. pharmacies.
What are the symptoms of a lonsuf?from lonsurf.com
Tell your healthcare provider right away if you get any of the following signs and symptoms of infection during treatment with LONSURF: fever, chills, or body aches.
What is a lonsuf?from lonsurfhcp.com
LONSURF is indicated for the treatment of adult patients with metastatic gastric or gastroesophageal junction adenocarcinoma previously treated with at least two prior lines of chemotherapy that included a fluoropyrimidine, a platinum, either a taxane or irinotecan, and if appropriate, HER2/neu‑targetedtherapy.
How long is a person alive after taking lonsufr?from lonsurf.com
In a clinical trial, half of the patients with metastatic colon or rectal cancer treated with LONSURF ® (trifluridine and tipiracil) tablets were still alive at 7.1 months and half of the patients who received placebo were still alive at 5.3 months. Worsening of the disease or death occurred in 88% of patients treated with LONSURF and 94% of patients who received placebo.
What happens if you take lonsuf?from lonsurf.com
LONSURF can cause a decrease in your white blood cells, red blood cells, and platelets. Low white blood cells can make you more likely to get serious infections that could lead to death. Your healthcare provider should do blood tests before you receive LONSURF, at day 15 during treatment with LONSURF, and as needed to check your blood cell counts. Your healthcare provider may lower your dose of LONSURF or stop LONSURF if you have low white blood cell or platelet counts
What is the molar ratio of Lonsurf?from lonsurfhcp.com
LONSURF®(trifluridine and tipiracil) tablets consist of a thymidine‑based nucleoside analog, trifluridine, and the thymidine phosphorylase inhibitor, tipiracil, at a molar ratio 1:0.5 (weight ratio, 1:0.471). Inclusion of tipiracil increases trifluridine exposure by inhibiting its metabolism by thymidine phosphorylase.
Is lonsurf safe for children?from lonsurf.com
a kind of stomach cancer called gastric cancer including cancer of the gastroesophageal junction that has spread to other parts of the body and who have been previously treated or cannot receive certain chemotherapy medications. It is not known if LONSURF is safe and effective in children.
Does lonsufra have side effects?from lonsurf.com
Tell your doctor if you have nausea, vomiting, or diarrhea that is severe or that does not go away. These are not all of the possible side effects of LONSURF.

Warnings and Precautions
- Severe Myelosuppression: LONSURF caused severe and life‑threatening myelosuppression (Grade 3‑4) consisting of neutropenia (38%), anemia (18%), thrombocytopenia (5%), and febrile neutropenia (3%). Two patients (0.2%) died due to neutropenic infection. A total of 12% of LONSURF‑treated patients received granulocyte‑colony stimulating factors. Obtain...
Use in Specific Populations
- Lactation:It is not known whether LONSURF or its metabolites are present in human milk. There are no data to assess the effects of LONSURF or its metabolites on the breast‑fed infant or the effects on milk production. Because of the potential for serious adverse reactions in breast‑fed infants, advise women not to breastfeed during treatment with LONSURF and for 1 day followin…
Adverse Reactions
- Most Common Adverse Drug Reactions in Patients Treated With LONSURF (≥5%):The most common adverse drug reactions in LONSURF‑treated patients vs placebo‑treated patients with mCRC, respectively, were asthenia/fatigue (52% vs 35%), nausea (48% vs 24%), decreased appetite (39% vs 29%), diarrhea (32% vs 12%), vomiting (28% vs 14%), infections (27% vs 16%), a…