The December 2017 OIG CIA was announced as part of a settlement between United Therapeutics and the Department of Justice (DOJ), which alleged that United Therapeutics induced patients to purchase its drugs by donating to charity PAPs over which it exerted some control.
Full Answer
What is the alleged violation of the OIG?
What is the OIG opinion on Caring Voice?
What is the OIG CIA?
Is Caring Voice Coalition offering financial assistance?
Is United Therapeutics in the CIA?
See 2 more
HHS OIG Approves Narrowly-Tailored Patient Assistance Program
On Jan. 15, 2020, the Department of Health and Human Services (HHS) Office of Inspector General (OIG) issued Advisory Opinion No. 20-02 which addresses whether a pharmaceutical manufacturer providing financial assistance to patients constitutes grounds for the imposition of sanctions under the civil monetary penalty provision prohibiting inducements to beneficiaries, section 1128A(a)(5) of the ...
Special Advisory Bulletin Provides Guidance On Patient Assistance ...
Washington, DC – HHS Inspector General Daniel R. Levinson today released a Special Advisory Bulletin providing guidance on the application of OIG fraud and abuse laws to patient assistance programs (PAPs) that offer assistance in obtaining outpatient prescription drugs to financially needy Medicare beneficiaries who enroll in the Medicare Part D drug benefit.
Advisory Opinion 20-02 | Office of Inspector General | Government ...
Updates. June 1, 2022. Requestor now seeks to modify AO 20-02 to include Requestor’s provision of financial assistance to eligible patients for travel, lodging, and certain other associated expenses in connection with a round trip to an approved treatment center for a cell-removal process associated with manufacturing Requestor’s drug.
Caring Voice Coalition, Mechanicsville, VA - Government of New York
Category Search Close Category Search. The Category Search is arranged by topic. Click on a category in the menu below to learn more about it. Use the location bar above to find providers of these services in your area.
K&L Gates - Increased Scrutiny of Patient Assistance Programs ...
In the midst of ongoing national debates regarding drug pricing [1] and access to innovative therapies, patient assistance programs (“PAPs”) are facing an evolving legal landscape, as well as increased enforcement scrutiny from regulators and legislators on a state and federal level.
Pharmaceutical Manufacturer Patient Assistance Program Information | CMS
Pharmaceutical manufacturers may sponsor patient assistance programs (PAPs) that provide financial assistance or drug free product (through in-kind product donations) to low income individuals to augment any existing prescription drug coverage.
What is OIG opinion 20-02?
On Jan. 15, 2020, the Department of Health and Human Services (HHS) Office of Inspector General (OIG) issued Advisory Opinion No. 20-02 which addresses whether a pharmaceutical manufacturer providing financial assistance to patients constitutes grounds for the imposition of sanctions under the civil monetary penalty provision prohibiting inducements to beneficiaries, section 1128A (a) (5) of the Social Security Act (the Act), the exclusion authority at section 1128 (b) (7) of the Act or the civil monetary penalty provision at section 1128A (a) (7) of the Act. These sections relate to the commission of acts described in section 1128B (b) of the Act, the federal anti-kickback statute.
What is an eligible patient?
Eligible patients are patients who have been prescribed the drug for an FDA-approved indication and have a household income that does not exceed 600 percent of the federal poverty level, who live more than two hours driving distance or 100 miles from the nearest center accepting patients and who have no insurance for non-emergency medical travel. The requestor offers the arrangement to eligible patients regardless of their provider or insurance status. To participate in the arrangement, the patient and caregiver (s) must agree not to request reimbursement from federal health care programs for costs covered under the arrangement. The requestor certified that it does not bill or otherwise shift the costs of the arrangement to the federal health care programs.
What is a drug infusion requestor?
Under the arrangement, the requestor assists eligible patients, between the ages of 18-25 years old, and up to two caregivers with travel, lodging, meals and certain out-of-pocket expenses they incur during and after the patient’s drug infusion. For patients 26 and older, the requestor provides the same support for a patient and one caregiver. The requestor does not provide assistance with patient travel or expenses associated with initial patient consultations, leukapheresis or follow-up visits beyond the post-infusion monitoring required by the drug’s prescribing information. The requestor does not authorize lodging under the arrangement to a patient treated by a center when the requestor has knowledge that the patient is eligible to receive lodging from the center, and such lodging is available for that patient’s use. The requestor also certified that it does not advertise the arrangement. Patients do not learn about, or become eligible for, the arrangement until they have been diagnosed with the appropriate disease and are prescribed treatment with the drug. Under the arrangement, the requestor provides reimbursement for gas and tolls or arranges for transportation via bus, rail, rental car or air travel for a patient and caregiver (s) to and from the closest center accepting patients using a third-party travel vendor.
How long does a patient have to be monitored after an infusion?
Patients receive assistance for four weeks post-infusion; however, if the patient’s physician determines that it is medically necessary to monitor the patient for risks of negative outcomes for longer than four weeks , the requestor provides assistance for the duration of monitoring deemed necessary by the physician.
What is a per discharge rate for a drug?
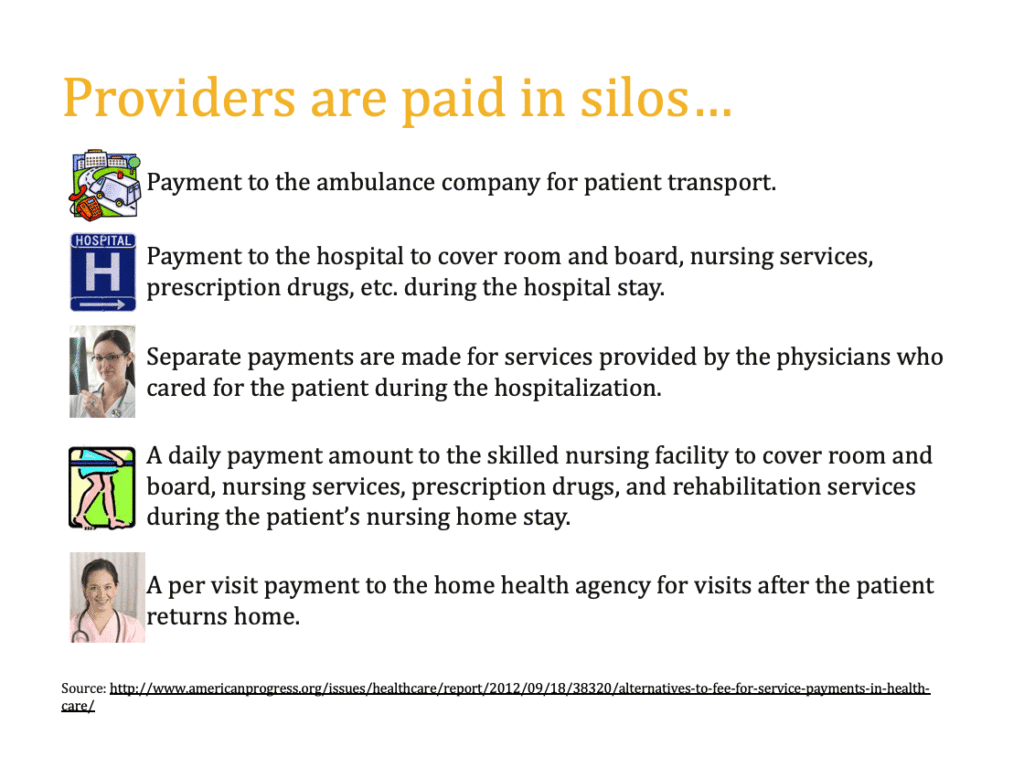
When inpatient hospitals infuse the drug, they receive a per-discharge payment rate based on the Medicare Severity Diagnosis Related Group (MS-DRG) to which the discharge is assigned. In addition to this payment, hospitals may also receive a New Technology Add-On Payment (NTAP) that will vary based on a hospital’s total inpatient covered charges and overall cost-to-charge ratio.
Can the OIG impose administrative sanctions?
The OIG advised that it will not impose administrative sanctions under the above-listed sections of the Act for the specific scenario described but noted that similar circumstances could create prohibited remuneration under the anti-kickback statute if the requisite intent to induce or reward referrals of federal health care program business were present.
Does a requestor authorize lodging under an arrangement?
The requestor does not authorize lodging under the arrangement to a patient treated by a center when the requestor has knowledge that the patient is eligible to receive lodging from the center, and such lodging is available for that patient’s use. The requestor also certified that it does not advertise the arrangement.
What is OIG 20-05?
OIG Advisory Opinion 20-05 represents another shot across the bow from the government to pharmaceutical manufacturers that attempt to subsidize federal health care program beneficiaries’ cost-sharing obligations for the manufacturers’ own drug products. This Advisory Opinion comes on the heels of recent federal enforcement activity surrounding pharmaceutical manufacturers’ involvement in patient assistance programs run by purportedly independent foundations. In those enforcement actions, the U.S. Department of Justice alleged that a number of pharmaceutical manufacturers violated the federal False Claims Act, 31 U.S.C. § 3729 et seq., by unlawfully paying the Medicare copayments for their own products through purportedly independent nonprofit charitable foundations that the manufacturers used as conduits. [1] The Department of Justice’s position on financial assistance provided directly to Medicare beneficiaries by pharmaceutical manufacturers is clear: such conduct not only violates the federal Anti-Kickback Statute, 42 U.S.C. § 1320a-7b (b), but also undermines the Medicare program’s copayment structure, which Congress intended to serve as a check against the prices manufacturers can charge for their drugs.
When did OIG issue advisory opinion 20-05?
On September 18, 2020, the U.S. Department of Health and Human Services’ Office of Inspector General (“OIG”) issued Advisory Opinion 20-05. In this unfavorable Advisory Opinion, OIG declined to approve a pharmaceutical manufacturer’s proposal to provide cost-sharing assistance directly to Medicare beneficiaries who are prescribed the manufacturer’s drugs.
Is OIG advisory opinion binding?
Although OIG Advisory Opinions are not binding on any individual or entity other than the requestor, they provide valuable insights into OIG’s interpretation of the law. Health care providers that provide financial assistance directly to federal health care program beneficiaries for the providers’ own items or services may wish to re-evaluate the fraud and abuse risks associated with such assistance in light of unfavorable Advisory Opinion 20-05 and the current enforcement environment. Consequently, the principles discussed in this Advisory Opinion may have ramifications well beyond the pharmaceutical industry.
Why are PAPs important?
Historically, the OIG has treated PAPs as important safety nets for patients who face chronic illnesses and high drug costs. The OIG issued a special advisory bulletin (SAB) in 2005 confirming that PAPs could help ensure patients had access to and could afford their medically necessary drugs. The OIG’s guidance evolved with its May 2014 SAB, which addressed the growing trend of independent charity PAPs establishing or operating specific disease funds that limit assistance to a subset of available products. The OIG articulated a concern with such PAPs, and indicated that it would view such programs as having a higher baseline risk of abuse when their assistance was limited to only a subset of available FDA-approved products for treatment of the disease. The OIG advised PAPs to define disease funds in accordance with widely recognized clinical standards and in a manner that covered a broad spectrum of products and manifestations of the disease ( e.g., without reference to specific symptoms, drug stages, treatment types, severity of symptoms or other “narrowing” factors).
Can PAPs be used to subsidize drug purchases?
As PAPs have the potential to be used by manufacturers to subsidize the purchase of their own products, or to improperly steer a patient’s drug selection, they can trigger scrutiny under the federal Anti-Kickback Statute (AKS) and Beneficiary Inducement Civil Monetary Penalty (CMP), among other laws.
When did the OIG change its advisory opinion?
Beginning in December 2015, the OIG modified five Advisory Opinions in order to update the analyses pursuant to certifications received. [9]
What is a rescinded OIG letter?
However, on November 28, 2017, the OIG issued a letter rescinding Advisory Opinion 06-04 (“Rescission Letter”), based on the charity’s “failure to fully, completely, and accurately disclose all relevant and material facts to OIG,” and CVC’s alleged failure to comply with certain factual certifications made to the OIG. Specifically, the OIG states that it determined that the charity “provided patient-specific data to one or more donors that would enable the donor (s) to correlate the amount and frequency of their donations with the number of subsidized prescriptions or orders for their products, and (ii) allowed donors to directly or indirectly influence the identification or delineation of Requestor’s disease categories.” [14] The Rescission Letter indicates that CVC’s failure to comply with the certifications “materially increased the risk” that CVC served as a conduit for financial assistance from a drug manufacturer donor to a patient, and thus inappropriate steerage to the donor’s drugs.
What is the rescission of advisory opinion 06-04?
Senate Finance Committee regarding the rescission of Advisory Opinion 06-04, including how the OIG came to learn about the misrepresentations that the charity made, and whether the OIG plans to audit or review other PAPs and similar advisory opinions.
What is the OIG opinion on CVC?
In December 2015, the OIG published a Modified Advisory Opinion 06-04, following the OIG’s request that CVC certify compliance with the additional factors outlined in the 2014 Special Advisory Bulletin. The Modified Advisory Opinion stated that CVC had certified compliance to each additional factor, and further that CVC had proposed additional modifications to its current operations. [13] The OIG concluded in the Modified Advisory Opinion 06-04 that CVC’s PAP was sufficiently low risk and the OIG would not impose CMPs or sanctions on CVC under the AKS.
What should stakeholders do with PAPs?
Stakeholders should also closely monitor federal and state legislative policy developments regarding PAPs, including copayment assistance and product coupons. K&L Gates regularly advises clients on health care fraud and abuse risk mitigation and compliance matters and facilitate stakeholder engagement with Congress and state legislators and HHS.
What are the two aspects of PAP?
The OIG has indicated that PAPs generally have two “remunerative aspects” that require scrutiny under the AKS: i) donor contributions , which the OIG stated can be analyzed as indirect remuneration to patients , and ii) financial assistance remuneration provided directly to patients. The OIG states that the AKS could be violated “if a donation is made to a PAP to induce the PAP to recommend or arrange for the purchase of the donor’s federally reimbursable items,” as well as if a PAP’s grant of financial assistance to a patient is made “to influence the patient to purchase (or induce the patient’s physician to prescribe) certain items.” [5]
What is the purpose of PAPs?
Department of Health and Human Services (“HHS”) Office of the Inspector General (“OIG”) has continually acknowledged that properly structured PAPs can provide important “safety net assistance” to patients with limited financial means who cannot afford necessary drugs. This Client Alert provides a comprehensive review ...
How many children have been helped by the Assistance Fund?
Since its founding in 2009, The Assistance Fund has helped nearly 135,000 children and adults access the treatment they need to stay healthy or manage a life-threatening, chronic, or rare disease. Learn more about how we serve our patients.
What is TAF provider portal?
TAF’s Provider Portal is a one-stop-shop for providers to see up-to-date patient information and so much more.
What is the OIG in PAP?
First, the OIG highlighted the autonomy of the charitable organization, the non-affiliation with any Donor, and the inability of any Donor to exert direct or indirect control or influence over the charitable organization or the Program. The requestor’s independent discretion to use donations was apparent in the facts discussed above, and the inability of Donors or their affiliates to influence the board of directors was adequately protected. Second, the OIG highlighted that under the requestor's PAP, patients must have selected their provider, practitioner, or supplier and have a treatment regimen in place prior to applying for benefits, and remain free to change them while receiving assistance. Further protection is found in the requestor certifying that it would not make referrals or recommendations to patients.
What are the protections afforded by the OIG?
First, eligibility decisions would be based solely on financial need, according to uniform standards applied consistently, without regard for the identity of the provider, practitioner, supplier, drug, device, referring party, or any Donor. Second, patients must have their provider, practitioner, or supplier, and their treatment plan, in place prior to applying for assistance (which the patient remains free to change while receiving assistance), and would receive support on a first-come, first-served basis. Eligibility decisions would not be based on whether the provider, practitioner, or supplier is a Donor, and the requestor would not make any referrals or recommendations or share Donor identities with patients. For these reasons, the OIG found the Program presented a low risk of fraud and abuse.
What is the OIG opinion 15-06?
In approving these disease funds, the OIG stressed the importance of the protection built into the arrangement, including the board of director independence from donors, strict limits on the involvement of donors and anyone related to them, the patients’ freedom of choice to switch providers and suppliers, and the requirement that patients select their providers and suppliers and have a treatment plan in place before applying to the PAP for assistance. Advisory Opinion 15-06 thus provides another concrete example of the type of PAP arrangement that the OIG is willing to approve.
What is the OIG's conclusion on donor contributions?
As to Donor contributions to the requestor, the OIG concluded that the design and administration of the Program presented minimal risk and would provide sufficient insulation to prevent assistance decisions being influenced by the Donors. The OIG based its analysis on four aspects of the arrangement that provide protection.
What are the two main aspects of the proposed arrangement?
As discussed below, the OIG considered two main aspects of the proposed arrangement: (1) Donor contributions to the requestor; and (2) the requestor’s assistance to patients.
What is OIG advisory opinion?
Department of Health & Human Services, Office of Inspector General (OIG) issued an advisory opinion approving a charitable organization’s proposal to provide financial assistance to individuals with chronic diseases by assisting with the costs of health insurance and drug and device therapies. In Advisory Opinion 15-06, the OIG drew upon its past guidance regarding patient assistance programs (PAPs) to approve an arrangement operated by a charity that involves disease funds under certain carefully defined parameters.
Can a requestor refer a patient to a physician?
The requestor would not refer patients or recommend or arrange for the use of any practitioner, provider, supplier, drug or device. Patients would use a benefit card at the patients' preferred pharmacy or device distributer if treatment is self-administered. Where treatment is physician-administered, the requestor would provide assistance directly to the patient’s physician or hospital, or directly to the patient (upon verification) if the physician or hospital does not accept third-party payments or the benefit card.
What is the alleged violation of the OIG?
According to the OIG, the alleged violations “materially increased the risk that [the charity] served as a conduit for financial assistance from a [drug company] donor to a patient,” and thus increased the risk that the charity’s Medicare patients would be steered to that company’s federally reimbursable drugs.
What is the OIG opinion on Caring Voice?
The Office of Inspector General’s (OIG) advisory opinion protected Caring Voice Coalition from liability under the Anti-Kickback Statute for its work providing financially needy Medicare patients with premium and cost-sharing assistance. Drug companies are the primary donors to almost all charity patient assistance programs (PAPs), including Caring Voice Coalition, while some drug companies also create their own charity PAPs. The OIG’s 2006 opinion issued to Caring Voice Coalition was fact-specific, as are all advisory opinions, and in this case was predicated on commitments the charity made to implement certain safeguards regarding contributions from donors and grants to beneficiaries.
What is the OIG CIA?
The December 2017 OIG CIA was announced as part of a settlement between United Therapeutics and the Department of Justice (DOJ), which alleged that United Therapeutics induced patients to purchase its drugs by donating to charity PAPs over which it exerted some control.
Is Caring Voice Coalition offering financial assistance?
Last week, one of the largest charity patient assistance programs in the country, Caring Voice Coalition, announced that it would not be offering financial assistance for any of its disease funds in 2018.
Is United Therapeutics in the CIA?
Subsequent to entering the CIA with United Therapeutics, and on the same day Caring Voice Coalition announced it would not offer financial assistance in 2018, OIG released a letter it sent to a drug company trade group, dated January 4, 2018.
