How much do PCSK9 inhibitors cost under Medicare Part D?
CMS Administrator Seema Verma, in a public letter to members of Congress in August, recognized that PCSK9 inhibitor prices dropped below the Part D specialty tier cost threshold ($670 per month in 2020), which she believed would allow Medicare beneficiaries to access the products on lower-cost tiers.
How does collaboration improve patient adherence to PCSK9 inhibitors?
Enhancing Healthcare Team Outcomes Due to the cost associated with a PCSK9 inhibitor, collaboration among physicians, clinical pharmacists, and specialty pharmacies is essential to ensure patient adherence to therapy.
Does insurance cover pcsk9i?
After insurance approval, the vast majority of patients successfully gained affordable access to PCSK9i, with only 2.3% unable to initiate or continue therapy because of cost (all of whom were Medicare patients).
What are PCSK9 inhibitors and how do they work?
PCSK9 inhibitors, a relatively new class of drugs designed to lower cholesterol levels and reduce the risk of heart attacks and strokes, have encountered challenges with uptake.
Is there a patient assistance program for Repatha?
RepathaReady® offers resources and support services to help patients stay on track with their high LDL treatment. Sign up today to see if you are eligible for the Repatha® Copay Card, and to receive nurse support, needle disposal containers, medication reminders and informational emails, and insurance assistance.
Does insurance cover PCSK9 inhibitors?
Note: PCSK9 Inhibitors are typically covered under pharmacy benefit plans. Certain prescription drugs require an authorization for coverage to ensure that appropriate treatment regimens are followed.
What is Allergan patient assistance program?
The Allergan Patient Assistance Program (PAP) provides Allergan medicines at no cost to eligible patients. If the patient qualifies, up to a twelve-month eligibility for the requested medication(s) or device(s) is approved for shipment to the patient's licensed prescriber for dispensing.
How much will PCSK9 inhibitors cost?
The drugs are expensive, costing over $12,000 a year. The major issues concern whether this type of therapy prolongs life and whether it is a good value. The point of view of the patient, health care system and society will influence value assessment.
Does Medicare pay for PCSK9 inhibitors?
However, a recent Avalere analysis of 2020 Part D formularies finds that, while all plans have removed PCSK9 inhibitors from the specialty tier, only a third of all Medicare Part D beneficiaries will be able to access those products on preferred brand tiers in 2020.
Is Repatha better than Praluent?
These studies have also shown that Repatha may be more effective at reducing LDL-C levels than Praluent when given in certain doses. Although more research is needed, the use of Repatha is also linked to increased HDL levels (the “good” cholesterol).
What is AbbVie Assistance Program?
myAbbVie Assist provides free medicine to qualifying patients. If you are uninsured or have limited insurance coverage, you may be eligible to receive prescribed AbbVie medication at no cost from our Program.
How do you qualify for free eliquis?
You may be eligible for the Free 30-Day Trial Offer for ELIQUIS® (apixaban) if:You have not previously filled a prescription for ELIQUIS;You have a valid 30-day prescription for ELIQUIS;You are being treated with ELIQUIS for an FDA-approved indication that an HCP has planned for more than 35 days of treatment;More items...
How do I contact AbbVie?
If you have questions, call us at 1-800-222-6885.
What are the side effects of PCSK9?
Side effects of PCSK9 inhibitorsflu-like symptoms such as cold, nausea, back and joint pain.soreness or itchiness where you give the injection.muscle pain.
How much do PCSK9 inhibitors lower cholesterol?
What are the benefits of PCSK9 inhibitors? PCSK9 inhibitors can significantly decrease LDL cholesterol. One study found that the medicines lower LDL cholesterol by about 50%.
Is PCSK9 a statin?
Two types of medications prescribed to treat high cholesterol include statins and PCSK9 inhibitors. Statins are a popular treatment that have been available since the 1980s. PCSK9 inhibitors, on the other hand, are a new type of cholesterol drug.
Who is a candidate for Repatha?
Eligible patients with high cholesterol (LDL-C ≥70 mg/dL or non-high-density lipoprotein cholesterol [non-HDL-C] ≥100 mg/dL) and established cardiovascular disease at more than 1,300 study locations around the world were randomized to receive Repatha subcutaneous 140 mg every two weeks or 420 mg monthly plus high- or ...
Does Cigna Cover Repatha?
Cigna covers evolocumab (Repatha®) as medically necessary when the following criteria are met for FDA Indications or Other Uses with Supportive Evidence: Prior Authorization is recommended for prescription benefit coverage of Repatha. All approvals are provided for the duration noted below.
How much do PCSK9 inhibitors lower cholesterol?
What are the benefits of PCSK9 inhibitors? PCSK9 inhibitors can significantly decrease LDL cholesterol. One study found that the medicines lower LDL cholesterol by about 50%.
What is PCSK9i therapy?
PCSK9i (protein convertase subtilisin/kexin type 9 inhibitors) are set to revolutionize the treatment of hypercholesterolemia in the management of atherosclerotic risk, but numerous reports have detailed unprecedented barriers to access for these drugs. To overcome these challenges, our group created a model to facilitate provision of this new therapy for patients who qualify according to Food and Drug Administration criteria. This report details the real-world follow-up experience of PCSK9i use in a large patient cohort structured to ensure rigor in data collection, analysis, and interpretation. The 271 patients approved and actively followed in our PCSK9i clinic between July 2015 and August 2018 represent a 97% approval rate from insurance, with 28% of prescriptions requiring at least one appeal. Over 50% of patients were statin intolerant. On average, there was a median lapse of 15 days between initial visit and insurance approval. PCSK9i therapy was affordable for most patients, with an average monthly out-of-pocket expense of $58.05 (median $0). Only 2.3% of patients were unable to initiate or continue therapy because of cost. Reductions from baseline in LDL (low-density lipoprotein) cholesterol and Lp (a) (lipoprotein [a])were comparable to published reports with median reductions of 60% and 23% at 1 year, respectively. PCSK9i therapy was well-tolerated overall, though 9% of patients reported adverse events, and 5% of patients discontinued due mostly to musculoskeletal and flu-like symptoms. Our practice model demonstrates that PCSK9i therapy can be accessed easily and affordably for the majority of eligible patients, resulting in dramatic improvement in lipid profile results. Moreover, our registry data suggest that results from the prospective clinical trials of PCSK9i on LDL and Lp (a) reduction and on tolerability are applicable to a real-world cohort.
What is PCSK9i?
During the initial PCSK9i visit, a clinical pharmacist and a physician assistant performed a medical history and physical exam that included detailed documentation of current medications, past lipid-lowering therapies (allopathic and naturopathic), reported intolerances or side effects, diagnoses of lipid disorders using insurance-guided documentation requirements, instruction on injection technique, and a prescription for the PCSK9i medication in appropriately selected patients. The PCSK9i prescription was sent to our specialty pharmacy to process and initiate an electronic prior authorization, which was then completed by either the physician assistant or clinical pharmacist. Once approved, the PCSK9i prescription was routed to the preferred pharmacy and the clinical pharmacist followed up with the patient to review medication cost, injection technique, potential side effects, and timeline for follow-up lipid testing. Follow-up with the physician assistant was scheduled for 6 weeks (within 5 days from the third injection), 6 months, and every 6 months thereafter. A blood sample was also collected before initiation of therapy (baseline). Measurement of plasma lipid and Lp (a) (lipoprotein [a]) concentrations was performed as described previously. 19
How many patients were in the PCSK9i cohort?
The cohort consists of 271 patients approved and actively treated with a PCSK9i. The average age was 61.8 years and the study population was equally divided between men and women ( Table 1 ). Nearly half of the cohort (46%) had atherosclerotic cardiovascular disease as qualifying diagnosis, whereas familial hypercholesterolemia alone or in combination with atherosclerotic cardiovascular disease made up the remaining indications (26% and 28%, respectively; Table 1 ). Fifty-three percent of subjects in this cohort were deemed statin intolerant (unable to tolerate at least 2 statins, one at the lowest therapeutic daily dose). Among patients with familial hypercholesterolemia, 46% were statin intolerant.
How long does it take to get PCSK9I approved?
The approval rate for PCSK9i was 97% overall, with 29% of patients requiring at least one insurance appeal before approval. The median time to approval was 15 days, whereas the median time to first injection was 38 days. Both timelines were significantly reduced over the 3-year experience as the PCSK9i workflow was optimized ( Table 5 ).
Who is Dr Shapiro supported by?
Dr Shapiro is supported by National Institutes of Health K12HD04 3488.
Is PCSK9i a statin?
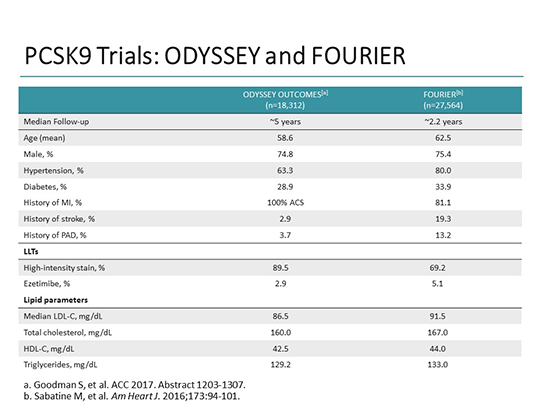
Results from landmark clinical outcome trials of PCSK9i (proprotein convertase subtilisin/kexin type 9 inhibitors) have confirmed the initial excitement for this class of LDL-C (low-density lipoprotein-cholesterol) lowering agents, 1–3 and the statin hypothesis has been supplanted by the LDL hypothesis. Hence, guideline recommendations and consensus statements now endorse the use of PCSK9i as appropriate second or third line agents, or as alternative therapy in cases of complete statin intolerance, for patients with established atherosclerotic cardiovascular disease or familial hypercholesterolemia with persistent hypercholesterolemia. 1–3 As evidence supporting their ability to improve cardiovascular outcomes continues to mount, PCSK9i use will undoubtedly continue to expand given the size of the target population.
What is PCSK9?
Proprotein convertase subtilisin/kexin type 9 (PCSK9) recently has emerged as an important regulator of cholesterol metabolism. Increased activity is associated with higher LDL-cholesterol levels, and certain gain of function mutations cause autosomal dominant familial hypercholesterolemia with very high cholesterol levels, premature atherosclerotic vascular disease, and the development of tendon xanthomas. Those with reduced PCSK9 activity, whether due to genetic polymorphism or administration of monoclonal antibodies to PCSK9, have lower cholesterol levels and a reduced risk of cardiovascular disease.
What is the treatment for hyperlipidemia?
As an adjunct to diet, alone or in combination with other lipid-lowering therapies (e.g., statins, ezetimibe), for the treatment of adults with primary hyperlipidemia (including heterozygous familial hypercholesterolemia) to reduce LDL-cholesterol
How many ml is a single use infusor?
Supplied as 140 mg/mL single-use prefilled syringe or autoinjector or 420 mg/3.5 mL solution in a single-use on-body infusor with a prefilled cartridge.
What is NCBI bookshelf?
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Does PCSK9 bind to LDL?
PCSK9, a product of hepatocytes, is secreted into the plasma, where it binds to the LDL receptor resulting in lysosomal degradation of the receptor. Thus, PCSK9 reduces the expression of LDL receptors on the cell membrane, thereby decreasing the clear ance of LDL-cholesterol.
What are the barriers to PCSK9?
1 Barriers to treatment with PCSK9 inhibitors, including prior authorization requirements and high out-of-pocket costs, may limit patient access. 2 Insurance approval can be delayed, and several appeals and resubmissions may be needed; however, even after obtaining approval, patients may not pick up their prescriptions from the pharmacy, mainly because of the high out-of-pocket costs. 2
Is Repatha contraindicated?
Contraindication: Repatha ® is contraindicated in patients with a history of a serious hypersensitivity reaction to Repatha ®. Serious hypersensitivity reactions including angioedema have occurred in patients treated with Repatha ®.
Praluent Prices
The cost for Praluent subcutaneous solution (150 mg/mL) is around $479 for a supply of 2 milliliters, depending on the pharmacy you visit. Prices are for cash paying customers only and are not valid with insurance plans.
Drugs.com Printable Discount Card
The free Drugs.com Discount Card works like a coupon and can save you up to 80% or more off the cost of prescription medicines, over-the-counter drugs and pet prescriptions.
Praluent Coupons and Rebates
Praluent offers may be in the form of a printable coupon, rebate, savings card, trial offer, or free samples. Some offers may be printed right from a website, others require registration, completing a questionnaire, or obtaining a sample from the doctor's office.
Patient Assistance Programs for Praluent
Patient assistance programs (PAPs) are usually sponsored by pharmaceutical companies and provide free or discounted medicines to low income or uninsured and under-insured people who meet specific guidelines. Eligibility requirements vary for each program.
