What is the Rituxan immunology co-pay program?
Rituxan Immunology Access Solutions can refer eligible patients to the Rituxan Immunology Co-pay Program for help with the out-of-pocket costs associated with Rituxan.* For eligible patients with commercial or public health insurance, Rituxan Immunology Access Solutions offers referrals to independent co-pay assistance foundations.†
How can I get help paying for Rituxan?
Visit Genentech-Access.com/RITUXAN. Genentech offers the Genentech Oncology Co-pay Assistance Program that may help you with the out-of-pocket costs of RITUXAN.*
What is Genentech access solutions for Rituxan?
Genentech Access Solutions is a program that helps people who are taking RITUXAN. Your health insurance plan and the cost of your medicine might keep you from getting your prescribed treatment. We may be able to help. To learn more about Genentech Access Solutions, call (888) 249-4918 or visit Genentech-Access.com/RITUXAN.
Is Rituxan available without a prescription?
RITUXAN is available by prescription only. What is RITUXAN? Non-Hodgkin’s Lymphoma (NHL): alone or with other chemotherapy medicines. Chronic Lymphocytic Leukemia (CLL): with the chemotherapy medicines fludarabine and cyclophosphamide. It is not known if RITUXAN is safe and effective in children with CLL.
How do you feel after Rituxan?
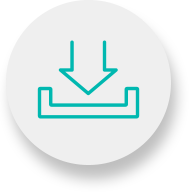
After a Rituxan (rituximab) infusion you may experience certain side effects or adverse reactions that make you feel unwell. Rituxan affects different people in different ways, but the more common side effects include: Fever (high temperature), muscle aches, headaches and chills, which are signs of infection.
What is Genentech Patient Foundation?
The Genentech Patient Foundation gives free Genentech medicine to people who don't have insurance coverage or who have financial concerns and meet eligibility criteria.
What is Rituxan used for?
RITUXAN® (rituximab) is a prescription medicine used to treat: Adults with Non-Hodgkin's Lymphoma (NHL): alone or with other chemotherapy medicines. Adults with Chronic Lymphocytic Leukemia (CLL): with the chemotherapy medicines fludarabine and cyclophosphamide.
What company makes rituximab?
Rituxan (Rituximab) is a monoclonal antibody indicated for treating advanced follicular lymphoma. The drug was developed by Biogen Idec and is co-promoted by Genentech, a subsidiary of Roche Group.
What drugs does Genentech make?
Our Medicines & ProductsMedicines.Actemra® (TOCILIZUMAB [authorized for Emergency Use])Actemra® (tocilizumab)Activase® (alteplase)Alecensa® (alectinib)Avastin® (bevacizumab)Boniva Tablets® (ibandronate sodium)Cathflo Activase® (alteplase)More items...
Who makes Valganciclovir?
Genentech: Valcyte® (valganciclovir hydrochloride) - Information for Patients.
How long can you stay on Rituxan?
Rituxan can provide up to 6 months of symptom improvement from 1 course of treatment (2 infusions given 2 weeks apart). Be sure to talk with your doctor to find out if starting and continuing treatment with Rituxan is right for you.
Do you lose your hair with Rituxan?
Hair loss. Hair loss is a possible side effect of Rituxan. However, in clinical trials, hair loss occurred only in people who took Rituxan for pemphigus vulgaris (PV). PV is a condition in which your skin and mucous membranes develop serious and painful blisters.
What is the success rate of rituximab?
Even with a short follow-up, overall survival rates improved in the group receiving chemotherapy and rituximab (P = 0.016)....Table 1.StudyRegimenEfficacyForstpointner, 200427R-FCM vs. FCMORR: 79% vs. 58%* Median PFS: 16 months vs. 10 months* OS (2 years): 90% vs. 70%7 more rows
Why am I so tired after Rituxan?
Serious side effects that can occur with Rituxan include: tumor lysis syndrome (nausea, vomiting, diarrhea, and tiredness that happen due to tumor cells breaking down and releasing their contents into your blood)
How many Rituxan treatments can a person have?
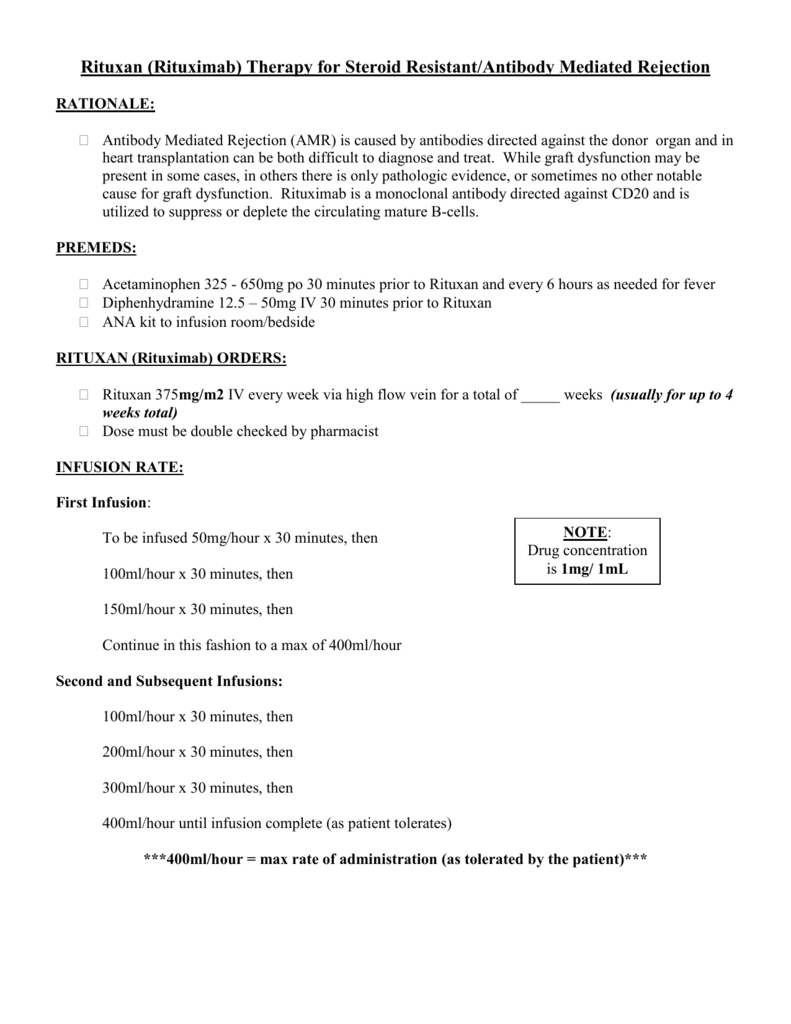
Administer RITUXAN as a single-agent every 8 weeks for 12 doses. Following completion of 6-8 cycles of CVP chemotherapy, administer once weekly for 4 doses at 6-month intervals to a maximum of 16 doses. Administer on Day 1 of each cycle of chemotherapy for up to 8 infusions.
Why are steroids given with Rituxan?
Rituximab injection is used together with steroids to treat granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA). These are immune disorders that cause blood vessels to be inflamed.
What is Genentech famous?
Genentech is a biotechnology company dedicated to pursuing groundbreaking science to discover and develop medicines for people with serious and life-threatening diseases. Our transformational discoveries include the first targeted antibody for cancer and the first medicine for primary progressive multiple sclerosis.
Who acquired Genentech?
Roche Holding'sRoche Holding's agreement on Thursday to acquire full ownership of Genentech for $46.8 billion is the third big drug industry merger this year.
Where is Genentech headquarters?
South San Francisco, CAGenentech / HeadquartersGenentech's corporate headquarters are in South San Francisco, California (37.657°N 122.379°W), with additional manufacturing facilities in Vacaville, California; Oceanside, California; and Hillsboro, Oregon.
Who started Genentech?
Robert A. SwansonHerbert BoyerGenentech/Founders
Where to report RITUXAN side effects?
You may report side effects to the FDA at (800) FDA-1088 or www.fda.gov/medwatch . You may also report side effects to Genentech at (888) 835-2555.
What to tell your healthcare provider before taking Rituximab?
Before receiving RITUXAN, tell your healthcare provider if you: Have had a severe reaction to RITUXAN or a rituximab product. Have a history of heart problems, irregular heartbeat, or chest pain. Have lung or kidney problems. Have had an infection, currently have an infection, or have a weakened immune system.
How long after a last dose of Rituxan can you give birth?
Females who are able to become pregnant should use effective birth control (contraception) during treatment with RITUXAN and for 12 months after the last dose of RITUXAN.
What to do before a Rituxan infusion?
Your healthcare provider should give you medicines before your infusion of RITUXAN to decrease your chance of having a severe infusion-related reactions. Tell your healthcare provider or get medical help right away if you get any of these symptoms during or after an infusion of RITUXAN: Hives (red itchy welts) or rash.
How long after a Rituxan can you breastfeed?
Are breastfeeding or plan to breastfeed. It is not known if RITUXAN passes into your breast milk. Do not breastfeed during treatment and for at least 6 months after your last dose of RITUXAN. Are taking any medications, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How long after a Rituxan infusion do you have a reaction?
Serious infusion-related reactions can happen during your infusion or within 24 hours after your infusion of RITUXAN.
What does it feel like to have a rituxan?
Severe Skin and Mouth Reactions: Tell your healthcare provider or get medical help right away if you get any of these symptoms at any time during your treatment with RITUXAN: Painful sores or ulcers on your skin, lips, or in your mouth. Blisters.
How long after Rituxan infusion can you have a reaction?
Infusion-Related Reactions: Infusion-related reactions are very common side effects of Rituxan treatment. Serious infusion-related reactions can happen during your infusion or within 24 hours after your infusion of Rituxan. Your healthcare provider should give you medicines before your infusion of Rituxan to decrease your chance of having a severe infusion-related reaction.
How to report side effects of Genentech?
Call your healthcare provider for medical advice about side effects. You may report side effects to the FDA at (800) FDA‐1088 or www.fda.gov/medwatch. You may also report side effects to Genentech at (888) 835‐2555.
Can you test if you are pregnant before taking Rituxan?
Your healthcare provider should do a pregnancy test to see if you are pregnant before starting Rituxan.
Does Rituxan cause heart problems?
Heart Problems: Rituxan may cause chest pain, irregular heartbeats, and heart attack. Your healthcare provider may monitor your heart during and after treatment with Rituxan if you have symptoms of heart problems or have a history of heart problems. Tell your healthcare provider right away if you have chest pain or irregular heartbeats during treatment with Rituxan
What is the rate of serious infections with Rituxan?
In the experience with Rituxan in 2578 RA patients, the rate of serious infection was 4.31 per 100 patient-years. The most common serious infections (≥0.5%) were pneumonia or lower respiratory tract infections, cellulitis, and urinary tract infections. Fatal serious infections included pneumonia, sepsis, and colitis. Rates of serious infection remain stable in patients receiving subsequent courses.
How long before a course of Rituxan can you administer non live shots?
For patients treated with Rituxan, physicians should review the patient’s vaccination status and patients should, if possible, be brought up to date with all immunizations in agreement with current immunization guidelines prior to initiating Rituxan and administer non-live vaccines at least 4 weeks prior to a course of Rituxan.
How long after Rituxan infusion do you have an adverse reaction?
Infusion-Related Reactions: In the Rituxan RA pooled, placebo-controlled studies, incidence of any adverse event within 24 hours of an infusion was 32% vs 23% after the first infusion, and 11% vs 13% after the second infusion in the Rituxan-treated patients and placebo group, respectively. Incidence of acute infusion-related reactions was 27% vs 19% after the first infusion, 9% vs 11% after the second infusion in the Rituxan-treated patients and placebo group, respectively. Serious acute infusion-related reactions were experienced by <1% of patients in either treatment group. Acute infusion-related reactions required dose modification (stopping, slowing, or interruption of the infusion) in 10% and 2% of patients receiving Rituxan or placebo, respectively, after the first course.
What is the most common infection with Rituxan?
The most common serious infection was pneumonia. Hypogammaglobulinemia: Hypogammaglobulinemia (IgA, IgG, or IgM below the lower limit of normal) has been observed in patients with GPA and MPA treated with Rituxan in GPA/MPA Study 1.
How long does it take for a patient to reactivate HBV?
HBV reactivation has been reported up to 24 months following completion of Rituxan therapy.
How long does it take for a PV to test positive for Rituximab?
Using a new ELISA assay, a total of 19/34 (56%) patients with PV treated with the Ritux 3 regimen tested positive for anti-rituximab antibodies by 18 months. The clinical relevance of anti-rituximab antibody formation in Rituxan treated PV patients is unclear.
How many patients tested positive for anti-rituximab?
A total of 273/2578 (11%) patients with RA tested positive for anti-rituximab antibodies at any time after receiving Rituxan. Anti-rituximab antibody positivity was not associated with increased infusion-related reactions or other adverse reactions. Upon further treatment, the proportions of patients with infusion-related reactions were similar between anti-rituximab antibody positive and negative patients, and most reactions were mild to moderate. Four anti-rituximab antibody positive patients had serious infusion-related reactions, and the temporal relationship between anti-rituximab antibody positivity and infusion-related reaction was variable. The clinical relevance of anti-rituximab antibody formation in Rituxan-treated patients is unclear.
What is rituximab used for?
Rituximab is a type of medication called a monoclonal antibody. It is used to treat certain types of cancer (e.g., non- Hodgkin's lymphoma).
Can rituximab cause heart problems?
WARNING: Rarely, serious (even fatal) side effects have occurred with rituximab use. Side effects such as breathing trouble, chest pain, irregular heartbeats, or a large change in amount of urine must be reported to your doctor immediately. Your doctor may stop rituximab or use additional treatment. Laboratory tests (e.g., electrolytes, kidney function) may be performed to monitor your progress. If you have heart or lung problems, a large number of cancer cells (above 25,000/mm3) in the blood, or certain cancers (CLL or lymphoma), you should be closely monitored.
How to report Rituxan side effects?
These are not all of the possible side effects with Rituxan. Call your healthcare provider for medical advice about side effects. You may report side effects to the FDA at (800) FDA-1088 or www.fda.gov/medwatch. You may also report side effects to Genentech at 1-888-835-2555.
How old do you have to be to take Rituxan?
Rituxan is not indicated in children less than 2 years of age with GPA or MPA or in children with conditions other than GPA or MPA.
What are the side effects of Rituxan?
infections (may include fever, chills) body aches. tiredness. nausea. In patients with GPA or MPA, the most common side effects of Rituxan also include: low white and red blood cells. swelling. diarrhea. muscle spasms.
How long after Rituxan is given can you get pregnant?
Females who are able to become pregnant should use effective birth control (contraception) during treatment with Rituxan and for 12 months after the last dose of Rituxan.
How long after Rituxan can you breastfeed?
are breastfeeding or plan to breastfeed. Patients should not breastfeed during treatment and for at least 6 months after the last dose of Rituxan. are taking any medications, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
What is the best medicine for RA?
Adults with Rheumatoid arthritis (RA): with another prescription medicine called methotrexate, to reduce the signs and symptoms of moderate to severe active RA, after treatment with at least one other medicine called a tumor necrosis factor (TNF) antagonist has been used and did not work well enough.
Can you take Rituxan with a heart attack?
People with serious infections should not receive Rituxan. Heart Problems: Rituxan may cause chest pain, irregular heartbeats, and heart attack. Your healthcare provider may monitor your heart during and after treatment with Rituxan if you have symptoms of heart problems or have a history of heart problems.
