What are the side effects of using Saphnelo?
How does Saphnelo work?
What is Saphnelo™ (anifrolumab-fnia)?
What is the target population for receiving Saphnelo? Will it work for everyone?
Is there any co-pay or patient assistance program for Saphnelo?
What is the only treatment for lupus?
When was Saphnelo approved?
See 4 more
About this website
.png?format=750w)
Does Medicare pay for Saphnelo?
cover SAPHNELO Examples of government-paid insurance are Medicare and Medicaid. Terms of Use section on following page. ‡Patients are responsible for any cost associated with the infusion administration above the $100 per infusion administration provided by the program.
Is patient assistance program legitimate?
Patient assistance programs (PAPs) are usually sponsored by pharmaceutical manufacturers and are promoted as a safety net for Americans who have no health insurance or are underinsured.
What is Allergan patient assistance program?
The Allergan Patient Assistance Program (PAP) provides Allergan medicines at no cost to eligible patients. If the patient qualifies, up to a twelve-month eligibility for the requested medication(s) or device(s) is approved for shipment to the patient's licensed prescriber for dispensing.
How do you infuse Saphnelo?
SAPHNELO WILL BE ADMINISTERED BY YOUR HEALTHCARE TEAM. Your healthcare provider will give you SAPHNELO through a needle placed in a vein (IV or intravenous infusion). The infusion takes about 30 minutes to give you the full dose of SAPHNELO. SAPHNELO is usually given 1 time every 4 weeks.
How do you qualify for free eliquis?
You may be eligible for the Free 30-Day Trial Offer for ELIQUIS® (apixaban) if:You have not previously filled a prescription for ELIQUIS;You have a valid 30-day prescription for ELIQUIS;You are being treated with ELIQUIS for an FDA-approved indication that an HCP has planned for more than 35 days of treatment;More items...
How do patient support programs work?
A patient assistance or support programs (PAPs or PSPs) exist to get you timely access to medication and to help you stay on track of your therapy. Being diagnosed with a complex disease or condition may come with unexpected financial burden and a need to better understand treatment options and next steps.
Does AbbVie have a patient assistance program?
myAbbVie Assist. We believe that people who need our medicines should be able to get them. That's why myAbbVie Assist provides free AbbVie medicine to qualifying patients. If you have any questions, visit the FAQs or call us at 1-800-222-6885.
What is my AbbVie assist?
myAbbVie Assist provides free medicine to qualifying patients. If you are uninsured or have limited insurance coverage, you may be eligible to receive prescribed AbbVie medication at no cost from our Program.
How do I contact AbbVie?
If you have a question that isn't answered, please call us at 1-800-222-6885.
What is the newest treatment for lupus?
Lupkynis (voclosporin)—approved in January 2021, is the first oral medication FDA-approved for lupus nephritis. It works by helping to stop cells that cause inflammation in lupus nephritis, while protecting the kidneys from serious damage.
How long does it take Saphnelo to work?
How long does it take to work? Saphnelo begins working in your body to relieve symptoms of SLE as soon as you receive your first dose. However, it can take about 3 months for you to notice the full effects of the medication. Talk with your doctor to find out how soon your symptoms might ease.
Is Saphnelo chemotherapy?
Your SAPHNELO infusion treatment is not chemotherapy. SAPHNELO contains anifrolumab-fnia, which is in a group of medicines called monoclonal antibodies. SAPHNELO is a biologic therapy that targets type 1 interferon (IFN-1) activity, which plays a key role in how lupus attacks your body.
How does the pan foundation work?
What does PAN cover? Our 12-month grants offer financial assistance for out-of-pocket medication costs, including co-pays, health insurance premiums, and transportation costs associated with medical care. Co-pay funds: assistance with deductibles, co-pays, and coinsurance for medications.
What is PAP in pharmacy?
Pharmaceutical manufacturers may sponsor patient assistance programs (PAPs) that provide financial assistance or drug free product (through in-kind product donations) to low income individuals to augment any existing prescription drug coverage.
Is prescription Assistance 123 legitimate?
The answer is yes. We are a legitimate service that is offered to those who really need our help. Our employees always strive for excellence and treat confidentiality and HIPPA regulations with the highest importance, as it is our duty to uphold them on behalf of our clients.
What is GSK patient assistance program?
The GSK Patient Assistance Program provides certain GSK medicines at no cost to eligible applicants. Eligibility is based on household income and insurance status. Residents of the United States and District of Columbia may be eligible for both Vaccine and Non-Vaccine Medicines.
Saphnelo vs Benlysta vs Rituxan : r/lupus - reddit
I’ve been on Benlysta (plus HCQ plus CellCept plus steroids) for several years and I am not getting any better. I am exploring options and would love your experience or thoughts about switching from Benlysta to Rituxan or Saphnelo.
Saphnelo: Uses, Dosage, Side Effects, Warnings - Drugs.com
Generic name: anifrolumab-fnia Dosage form: injection, for intravenous use Drug class: Selective immunosuppressants Medically reviewed by Judith Stewart, BPharm.Last updated on Oct 20, 2021. Dosage; Side effects; Interactions; What is Saphnelo? Saphnelo is a prescription medicine used to treat adults with moderate to severe systemic lupus erythematosus (SLE or lupus) who are receiving other ...
FDA Approves New Lupus Treatment - Verywell Health
People taking Saphnelo have a higher risk for viral infections and need to take precautions against COVID-19. “During the trial, researchers were carefully monitoring COVID-19 infection rates, and they did not see a signal suggesting higher rates of infection with the drug," says Susan Manzi, MD, MPH, a rheumatologist who was involved in Saphnelo clinical trials.
SAPHNELO FAQ - Lupus Society of Illinois
What is SAPHNELO and how does it work? SAPHNELO is a first-in-class type I interferon (IFN) receptor antagonist, a human monoclonal antibody that binds to subunit 1 of the type I IFN receptor, blocking the activity of type I IFNs.1 Type I IFN are cytokines (cell signaling proteins) involved in a key pathway in lupus pathogenesis.2 Up to 80% of adult patients with moderate-to-severe systemic ...
FDA Approves Saphnelo: First New Systemic Lupus Erythematosus ... - GoodRx
Last week, AstraZeneca announced that the FDA approved Saphnelo (anifrolumab-fnia), a first-in-class medication for adults with moderate to severe systemic lupus erythematosus (SLE or “lupus”) who are receiving standard therapy. There are a few different types of lupus, but SLE — inflammation that affects multiple organs or organ systems — is the most common.
SAPHNELO (anifrolumab) approved in the US for moderate to severe ...
WILMINGTON, Del., August 2, 2021 – AstraZeneca’s SAPHNELO™ (anifrolumab-fnia) has been approved in the US for the treatment of adult patients with moderate to severe systemic lupus erythematosus (SLE) who are receiving standard therapy.
Saphnelo Prices
The cost for Saphnelo intravenous solution (300 mg/2 mL) is around $4,812 for a supply of 2 milliliters, depending on the pharmacy you visit. Prices are for cash paying customers only and are not valid with insurance plans.
Drugs.com Printable Discount Card
The free Drugs.com Discount Card works like a coupon and can save you up to 80% or more off the cost of prescription medicines, over-the-counter drugs and pet prescriptions.
Saphnelo Coupons and Rebates
Saphnelo offers may be in the form of a printable coupon, rebate, savings card, trial offer, or free samples. Some offers may be printed right from a website, others require registration, completing a questionnaire, or obtaining a sample from the doctor's office.
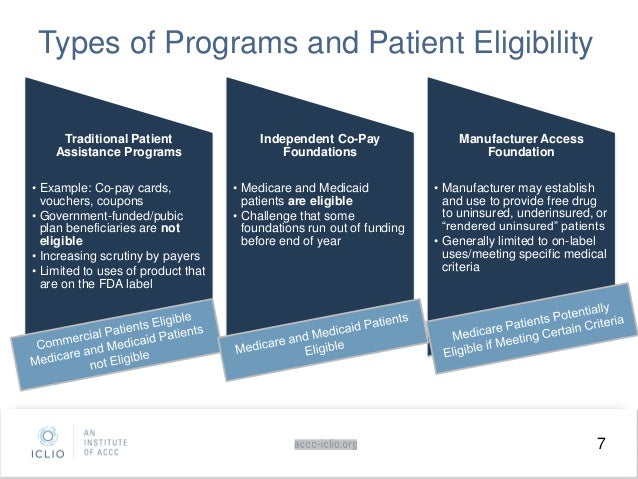
Patient Assistance Programs for Saphnelo
Patient assistance programs (PAPs) are usually sponsored by pharmaceutical companies and provide free or discounted medicines to low income or uninsured and under-insured people who meet specific guidelines. Eligibility requirements vary for each program.
What is the phone number for the SAPHNELO program?
Patient must be enrolled in the program before use. See full Eligibility and Terms of Use at www.SAPHNELO.com or call Access 360 at 1-866-SAPHNELO ( 1-866-727-4635), Monday–Friday, 8 AM to 8 PM ET, excluding holidays.
What insurance is required for SAPHNELO?
Patients must have commercial health insurance and a valid prescription for SAPHNELO. Patients who are enrolled in a state or federally funded prescription insurance program are not eligible for this offer. This includes patients enrolled in Medicare Part B, Medicare Part D, Medicaid, Medigap, Veterans Affairs (VA), Department of Defense (DoD) programs or TriCare, and patients who are Medicare eligible and enrolled in an employer-sponsored group waiver health plan or government-subsidized prescription drug benefit program for retirees. Patients who are enrolled in a state or federally funded prescription insurance program may not use this program even if they elect to be processed as an uninsured (cash-paying) patient. This offer is not insurance and is restricted to residents of the United States and Puerto Rico. Patients who are residents of Massachusetts, Michigan, Minnesota, or Rhode Island are not eligible for infusion administration assistance.
How to contact Astrazeneca about access 360?
For more information and additional resources, visit: https://www.astrazeneca-us.com/medicines/Affordability.html or call an Access 360 representative at 1-866-SAPHNELO ( 1-866-727-4635).
What is SAPHNELO 360?
Access 360 is a part of SAPHNELO Supports and can help you find information about coverage, costs, and affordability programs.
Does SAPHNELO cover out of pocket costs?
The SAPHNELO™ (anifrolumab-fnia) infusion for Intravenous infusion, 300 mg Savings Program is for commercially insured patients to cover patient out-of-pocket costs for SAPHNELO and its infusion administration up to $16,500 per calendar year. Patients with commercial insurance where the health plan does NOT cover SAPHNELO may qualify for the Denied Co-pay Savings Program. Subject to eligibility and terms of use.
Does AstraZeneca have a prescription savings program?
AZ&Me Prescription Savings Program provides AstraZeneca medicines at no cost to qualifying patients.
What is SAPHNELO CO-PAY?
The SAPHNELO Co-pay Savings Program may help you pay a set amount of out-of-pocket costs.
How to contact Astrazeneca about access 360?
For more information and additional resources, visit: https://www.astrazeneca-us.com/medicines/Affordability.html or call an Access 360 representative at 1-866-SAPHNELO ( 1-866-727-4635).
Does SAPHNELO cover office visits?
The SAPHNELO Co-pay Savings Program covers the cost of the drug and administration but does not cover costs for office visits or any other associated costs.
What are the side effects of using Saphnelo?
The most frequent adverse events (side effects) in people who received Saphnelo in the three clinical trials included nasopharyngitis (commonly referred to as the cold), upper respiratory tract infections, bronchitis, infusion-related reactions, and herpes zoster (shingles). In the most recent (Phase 3) clinical trials, called TULIP-1 and -2 all cases of herpes zoster were only on the skin and most resolved with medication. None were severe enough to cause people to stop receiving Saphnelo. In patients taking Saphnelo there was no increase in serious infections or in overall serious adverse events.
How does Saphnelo work?
Saphnelo dampens the excessive type I interferon signature that is found in many lupus patients. Like many aspects of lupus, there is nothing intrinsically bad about type I interferon signals. Interferons are there for a good purpose, which is to signal the immune system to be activated when an infection is detected. In lupus, the balance has been destabilized so that too many unnecessary interferon signals are getting through leading to inflammation in different parts of the body that can cause a person to become ill. Saphnelo binds to the main receptor (a protein called the IFNAR receptor) that transmits the signals from type I interferons throughout the body. Saphnelo has been shown to dampen these signals and to improve the symptoms of lupus.
What is Saphnelo™ (anifrolumab-fnia)?
Saphnelo, a human monoclonal antibody, is a type 1 interferon (INF) receptor antagonist that inhibits a key protein in the immune system called the IFNAR receptor. This protein acts like a transmitter, amplifying signals from tiny messengers called type I interferons. This activates many parts of the immune system and can trigger major inflammation. Various studies have found that between 50% and 80% of adults with lupus, and up to 90% of children with the disease, have evidence of elevated type I interferon stimulation which is measured by a test called the interferon signature. This signature is associated with risk for more severe lupus features.
What is the target population for receiving Saphnelo? Will it work for everyone?
Saphnelo was developed for treating moderate to severe systemic lupus erythematosus (SLE). Data from clinical studies showed that people with lupus treated with Saphnelo added to their usual treatment had improvements in overall lupus symptoms, in skin lupus (rash), and joints, and in the ability to taper steroid doses.
Is there any co-pay or patient assistance program for Saphnelo?
Access and out-of-pocket costs are determined by individual health insurance coverage. For eligible, commercially insured people, AstraZeneca provides co-pay savings programs to help with costs at the pharmacy. And for people without coverage and those with Medicare/Medicaid who need extra help paying for Saphonelo, their healthcare provider can contact Access 360 at [1-833-360-HELP] to determine what options may be available.
What is the only treatment for lupus?
Saphnelo is the only available treatment for lupus that targets the type I interferon receptor IFNAR and inhibits signals from all the different type I interferons. Some of its effects on the immune imbalances in lupus are likely to be unique but other aspects of its impact may overlap with other treatments.
When was Saphnelo approved?
The U.S. Food and Drug Administration (FDA) approved Saphnelo to treat adults with moderate to severe systemic lupus erythematosus (SLE ) on August 2, 2021. SLE is the most common form of lupus. Saphnelo is the third therapy for lupus to receive regulatory approval since 2011. We have answered some of the most common questions we receive about Saphnelo below.
IMPORTANT SAFETY INFORMATION
Serious infections: SAPHNELO can lower the ability of your immune system to fight infections. You may be at a higher risk of developing respiratory infections and shingles (herpes zoster) during treatment with SAPHNELO. Infections could be serious, leading to hospitalization or death
What is SAPHNELO?
SAPHNELO is a prescription medicine used to treat adults with moderate to severe systemic lupus erythematosus (SLE or lupus) who are receiving other lupus medicines.
How Access 360 Can Help
Specialists are ready to help navigate coverage options, financial support, and patient support programs. Learn how to enroll today
Affording SAPHNELO
Explore the different financial support programs and eligibility requirements to help with SAPHNELO treatment costs and out-of-pocket expenses
What are the side effects of using Saphnelo?
The most frequent adverse events (side effects) in people who received Saphnelo in the three clinical trials included nasopharyngitis (commonly referred to as the cold), upper respiratory tract infections, bronchitis, infusion-related reactions, and herpes zoster (shingles). In the most recent (Phase 3) clinical trials, called TULIP-1 and -2 all cases of herpes zoster were only on the skin and most resolved with medication. None were severe enough to cause people to stop receiving Saphnelo. In patients taking Saphnelo there was no increase in serious infections or in overall serious adverse events.
How does Saphnelo work?
Saphnelo dampens the excessive type I interferon signature that is found in many lupus patients. Like many aspects of lupus, there is nothing intrinsically bad about type I interferon signals. Interferons are there for a good purpose, which is to signal the immune system to be activated when an infection is detected. In lupus, the balance has been destabilized so that too many unnecessary interferon signals are getting through leading to inflammation in different parts of the body that can cause a person to become ill. Saphnelo binds to the main receptor (a protein called the IFNAR receptor) that transmits the signals from type I interferons throughout the body. Saphnelo has been shown to dampen these signals and to improve the symptoms of lupus.
What is Saphnelo™ (anifrolumab-fnia)?
Saphnelo, a human monoclonal antibody, is a type 1 interferon (INF) receptor antagonist that inhibits a key protein in the immune system called the IFNAR receptor. This protein acts like a transmitter, amplifying signals from tiny messengers called type I interferons. This activates many parts of the immune system and can trigger major inflammation. Various studies have found that between 50% and 80% of adults with lupus, and up to 90% of children with the disease, have evidence of elevated type I interferon stimulation which is measured by a test called the interferon signature. This signature is associated with risk for more severe lupus features.
What is the target population for receiving Saphnelo? Will it work for everyone?
Saphnelo was developed for treating moderate to severe systemic lupus erythematosus (SLE). Data from clinical studies showed that people with lupus treated with Saphnelo added to their usual treatment had improvements in overall lupus symptoms, in skin lupus (rash), and joints, and in the ability to taper steroid doses.
Is there any co-pay or patient assistance program for Saphnelo?
Access and out-of-pocket costs are determined by individual health insurance coverage. For eligible, commercially insured people, AstraZeneca provides co-pay savings programs to help with costs at the pharmacy. And for people without coverage and those with Medicare/Medicaid who need extra help paying for Saphonelo, their healthcare provider can contact Access 360 at [1-833-360-HELP] to determine what options may be available.
What is the only treatment for lupus?
Saphnelo is the only available treatment for lupus that targets the type I interferon receptor IFNAR and inhibits signals from all the different type I interferons. Some of its effects on the immune imbalances in lupus are likely to be unique but other aspects of its impact may overlap with other treatments.
When was Saphnelo approved?
The U.S. Food and Drug Administration (FDA) approved Saphnelo to treat adults with moderate to severe systemic lupus erythematosus (SLE ) on August 2, 2021. SLE is the most common form of lupus. Saphnelo is the third therapy for lupus to receive regulatory approval since 2011. We have answered some of the most common questions we receive about Saphnelo below.
