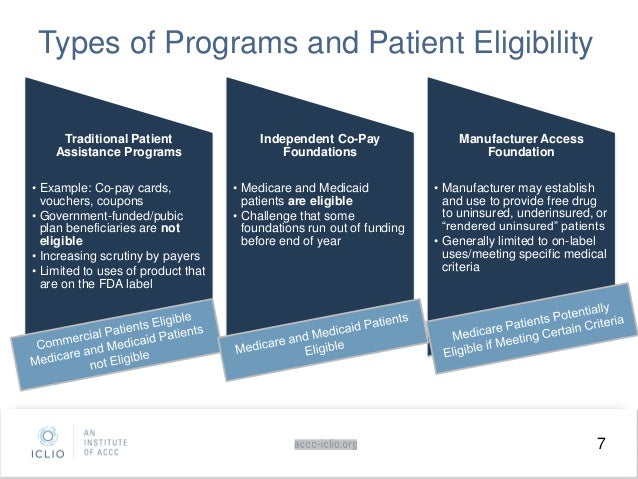
Patient Assistance Programs for Yescarta Patient assistance programs (PAPs) are usually sponsored by pharmaceutical companies and provide free or discounted medicines to low income or uninsured and under-insured people who meet specific guidelines. Eligibility requirements vary for each program.
Full Answer
What is Yescarta used to treat?
YESCARTA is a treatment for your non-Hodgkin lymphoma. It is used when you have failed at least two other kinds of treatment. YESCARTA is different than other cancer medicines because it is made from your own white blood cells, which have been modified to recognize and attack your lymphoma cells. A Chance for Complete Remission.
How do you treat CRS with Yescarta?
Prophylactic treatment with corticosteroids was administered to a cohort of 39 patients for 3 days beginning on the day of infusion of YESCARTA. Thirty-one of the 39 patients (79%) developed CRS and were managed with tocilizumab and/or therapeutic doses of corticosteroids with no patients developing ≥ Grade 3 CRS.
What is the mortality and morbidity associated with Yescarta treatment?
Neurologic toxicities (including immune effector cell-associated neurotoxicity syndrome (ICANS)) that were fatal or life-threatening occurred following treatment with YESCARTA Neurologic toxicities occurred in 78% (330/422) of patients with NHL receiving YESCARTA, including ≥ Grade 3 cases in 25%
What is Yescarta made from?
YESCARTA is made from your own white blood cells, which have been modified to recognize and attack your lymphoma cells. In a clinical study of 101 patients with non-Hodgkin lymphoma who had failed other treatments, YESCARTA was shown to help 51% (52 out of 101) achieve complete remission.

Who is eligible for Yescarta?
Yescarta is approved for use in adult patients with large B-cell lymphoma after at least two other kinds of treatment failed, including DLBCL, primary mediastinal large B-cell lymphoma, high grade B-cell lymphoma and DLBCL arising from follicular lymphoma.
What is Yescarta approved for?
The FDA approval of Yescarta CAR T-cell therapy for adult patients with large B-cell lymphoma (LBCL) that is refractory to first-line chemoimmunotherapy or that relapses within 12 months of first-line chemoimmunotherapy is based on results from the ZUMA-7 study.
What is Yescarta treatment?
YESCARTA is a prescription medicine used to treat two types of non-Hodgkin lymphoma: large B-cell lymphoma when your first treatment did not work or your cancer returned within a year of first treatment, OR when at least two kinds of treatment have failed to control your cancer.
What is the cost of Yescarta?
by Drugs.com The cost of Yescarta, as reported by the manufacturer, is $373,000 per treatment regimen.
Is Yescarta a cell therapy?
YESCARTA is the first CAR T-cell therapy approved for patients whose first treatment did not work or whose cancer returned within a year of first treatment.
Is Yescarta a car T therapy?
Made by Kite Pharma, Yescarta is a form of CAR-T therapy. With this novel type of immunotherapy, blood is drawn from a patient, T-cells (white blood cells that detect disease-causing organisms in the body) are extracted and genetically re-engineered.
Is Yescarta a one time treatment?
YESCARTA is administered as a single infusion at an Authorized Treatment Center.
Is CAR T-cell therapy FDA approved?
On February 28, the Food and Drug Administration (FDA) approved ciltacabtagene autoleucel (Carvykti) for adults with multiple myeloma that is not responding to treatment (refractory) or has returned after treatment (relapsed).
What is the difference between Yescarta and Tecartus?
While Tecartus shares the same design as Yescarta, also made by Kite Pharma, Inc., the difference lies in the manufacturing process. Tecartus undergoes a white blood cell enrichment process, which is necessary for certain types of B-cell blood cancers, such as MCL, where circulating lymphoblasts are a common feature.
How much does Luxturna treatment cost?
Official answer. The cost of Luxturna is $850,000 per a one-time treatment; however, the manufacturer states it is offering outcomes-based pricing and other innovative payment tools to lessen the cost of treatment to insurers and patients.
Is Yescarta approved in EU?
--(BUSINESS WIRE)-- Kite, a Gilead Company (Nasdaq: GILD), today announces that the European Commission (EC) has approved its CAR T-cell therapy Yescarta® (axicabtagene ciloleucel) for the treatment of adult patients with relapsed or refractory follicular lymphoma (FL) after three or more lines of systemic therapy.
Is Yescarta approved for follicular lymphoma?
Kite's CAR T-cell Therapy Yescarta® Granted European Marketing Authorization for the Treatment of Relapsed or Refractory Follicular Lymphoma.
What is the difference between Yescarta and Tecartus?
While Tecartus shares the same design as Yescarta, also made by Kite Pharma, Inc., the difference lies in the manufacturing process. Tecartus undergoes a white blood cell enrichment process, which is necessary for certain types of B-cell blood cancers, such as MCL, where circulating lymphoblasts are a common feature.
What is Kymriah approved for?
Basel, May 28, 2022 — Novartis today announced the US Food and Drug Administration (FDA) has granted accelerated approval for Kymriah® (tisagenlecleucel) for the treatment of adult patients with relapsed or refractory (r/r) follicular lymphoma (FL) after two or more lines of systemic therapy.
Is Tecartus FDA approved?
U.S. FDA Approves Kite's Tecartus® as the First and Only Car T for Adults With Relapsed or Refractory B-cell Acute Lymphoblastic Leukemia.