What are the factors that affect Indivior Group?
Is Perseris an injectable?
About this website

Is Perseris covered by insurance?
Perseris is currently not covered by any Medicare insurance plans. Without coverage, you may have to pay the full cash price of $2,638.95. Save on this total by using a SingleCare savings card.
Who owns Indivior?
Reckitt Benckiser GroupWhen Indivior was known as Reckitt Benckiser Pharmaceuticals, it was a subsidiary of British conglomerate Reckitt Benckiser Group (RB Group). RB Group paid $1.4 billion in 2019 to resolve its liability to the United States related to the marketing of Suboxone.
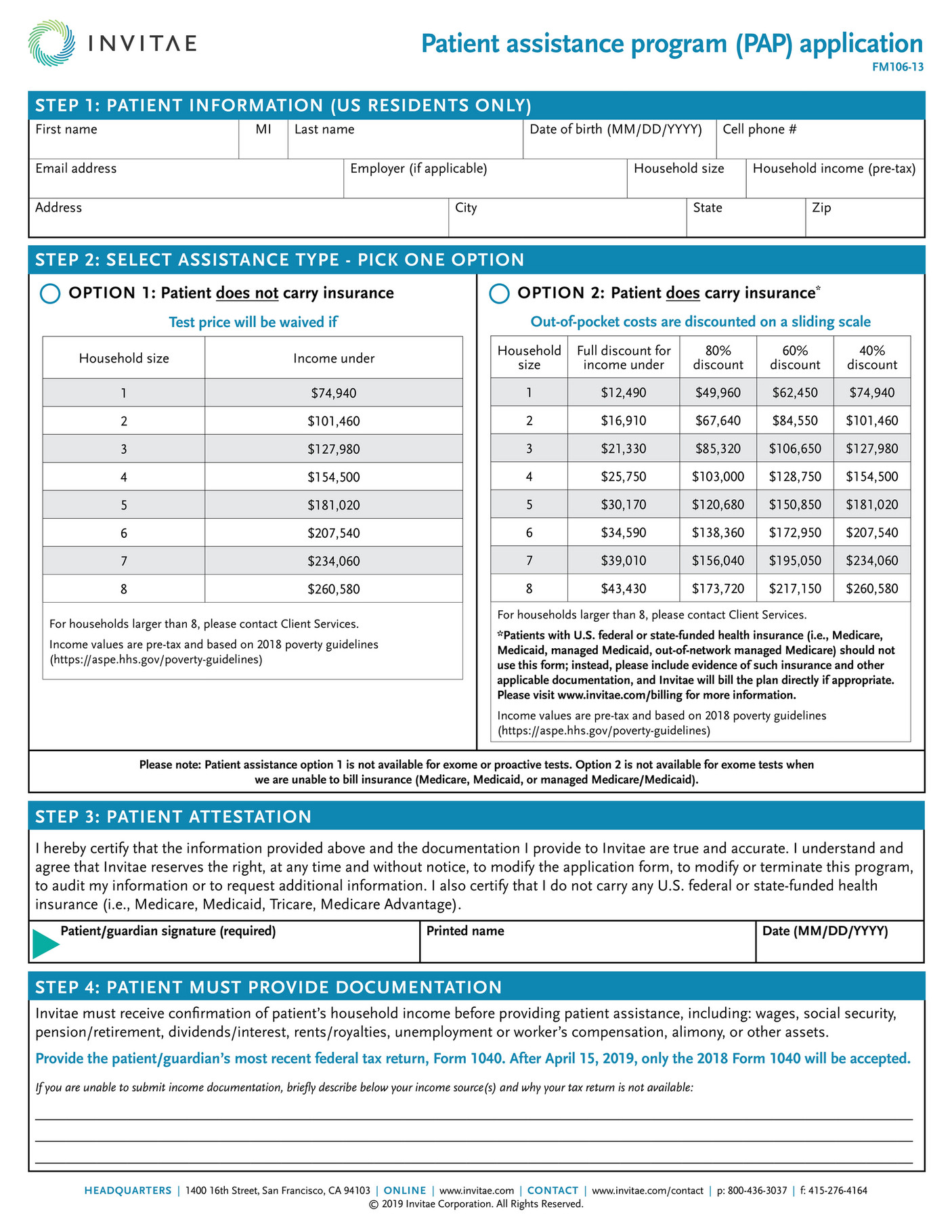
What is Allergan patient assistance program?
The Allergan Patient Assistance Program (PAP) provides Allergan medicines at no cost to eligible patients. If the patient qualifies, up to a twelve-month eligibility for the requested medication(s) or device(s) is approved for shipment to the patient's licensed prescriber for dispensing.
What drug company invented Suboxone?
Suboxone is Two-For-One Originally, a pharmaceutical company called Reckitt released buprenorphine in 1995 under the name Subutex.
Why is Suboxone being discontinued?
In addition, the government charged that Indivior announced it would discontinue its tablet form of Suboxone "based on supposed 'concerns regarding pediatric exposure' to tablets, despite Indivior executives' knowledge that the primary reason for the discontinuance was to delay the Food and Drug Administration's ...
Is Indivior being sued?
(Reuters) - Drugmaker Indivior Inc must face a lawsuit by 42 state attorneys general accusing it of using illegal tactics to shield its opioid addiction treatment Suboxone from generic competition, a federal judge has ruled.
How do you qualify for free eliquis?
You may be eligible for the Free 30-Day Trial Offer for ELIQUIS® (apixaban) if:You have not previously filled a prescription for ELIQUIS;You have a valid 30-day prescription for ELIQUIS;You are being treated with ELIQUIS for an FDA-approved indication that an HCP has planned for more than 35 days of treatment;More items...
Does AbbVie have a patient assistance program?
myAbbVie Assist. We believe that people who need our medicines should be able to get them. That's why myAbbVie Assist provides free AbbVie medicine to qualifying patients. If you have any questions, visit the FAQs or call us at 1-800-222-6885.
What is my AbbVie assist?
myAbbVie Assist provides free medicine to qualifying patients. If you are uninsured or have limited insurance coverage, you may be eligible to receive prescribed AbbVie medication at no cost from our Program.
What's going on with the Suboxone lawsuit?
The group of states accuse Indivior Inc. of illegally shifted the market for the drug, Suboxone, from tablets to film in an attempt to eradicate the demand for tablets. It's alleged that the company is doing this to preserve its monopoly on the drug.
How much will I get from the Suboxone settlement?
The agency is sending payments to people who were prescribed Suboxone film and filed a valid claim before the deadline. Claimants are receiving $35.61 for each month that they were prescribed Suboxone film between March 1, 2013 and February 28, 2019. If you receive a check, please cash it within 90 days.
How much are Suboxone strips worth?
Suboxone is a prescribed opioid blocker that is similar to methadone. “Monetary value on the street, $10 to $15 if they were to sell it privately, but each one of those strips inside the prison walls would go up to $400,” Azzara said.
Is Indivior a public company?
Indivior is a specialty pharmaceuticals business....Indivior.TypePublic companyKey peopleGraham Hetherington, Chairman Mark Crossley, CEOProductsSubutex/Subuxone/SublocadeRevenue$791 million (2021)8 more rows
Who is the CEO of Indivior?
Mark Crossley -Mark Crossley - Chief Executive Officer - Indivior | LinkedIn.
Is Indivior a good company?
Is Indivior a good company to work for? Indivior has an overall rating of 3.7 out of 5, based on over 199 reviews left anonymously by employees. 74% of employees would recommend working at Indivior to a friend and 79% have a positive outlook for the business. This rating has improved by 3% over the last 12 months.
When was Indivior founded?
1994INDIVIOR PLC / Founded
What are the factors that affect Indivior Group?
Various factors may cause differences between Indivior's expectations and actual results, including: factors affecting sales of Indivior Group’s products; the outcome of research and development activities; decisions by regulatory authorities regarding the Indivior Group’s drug applications ; the speed with which regulatory authorizations, pricing approvals and product launches may be achieved; the outcome of post approval clinical trials; competitive developments; difficulties or delays in manufacturing; the impact of existing and future legislation and regulatory provisions on product exclusivity ; trends toward managed care and healthcare cost containment; legislation or regulatory action affecting pharmaceutical product pricing , reimbursement or access; claims and concerns that may arise regarding the safety or efficacy of the Indivior Group’s products and product candidates; risks related to legal proceedings, including the ongoing investigative and antitrust litigation matters ; the Indivior Group’s ability to protect its patents and other intellectual property; the outcome of patent infringement litigation relating to Indivior Group’s products , including the ongoing ANDA lawsuits; changes in government al laws and regulations; issues related to the outsourcing of certain operational and staff functions to third parties; uncertainties related to general economic, political, business, industry, regulatory and market conditions; and the impact of acquisitions, divestitures, restructurings, internal reorganizations, product recalls and withdrawals and other unusual items.
Is Perseris an injectable?
Slough, UK and Richmond, VA, 27 February 2019 – Indivior PLC (LON: INDV) today announced the launch of once-monthly PERSERISTM (risperidone) for extended-release injectable suspension in the United States. PERSERIS is approved by the U.S. Food and Drug Administration for the treatment of schizophrenia in adults. PERSERIS delivers its active ingredient, risperidone, in an extended-release delivery system with no loading doses or oral supplementation recommended.
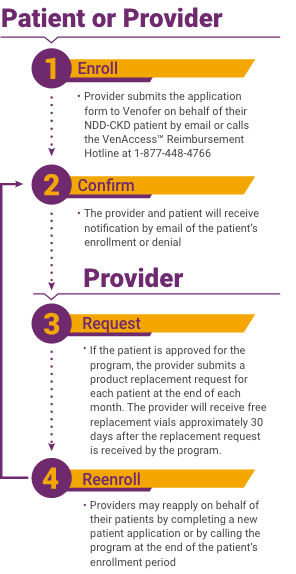
How to enroll in Insupport?
There are two ways to enroll a patient in the Program: 1 Online Enrollment#N#You may enroll an eligible patient online by going to the INSUPPORT® Portal located at www.insupportportal.com. If the patient is not in the office at the time of enrollment, you may request patient authorization from the Portal and the patient can complete and submit the form to INSUPPORT® via DocuSign. Once the patient authorization is received, INSUPPORT® can process the request. Please ensure both provider and patient read the Terms and Conditions for the INSUPPORT® Copay Assistance Program and provide the required electronic signature and date. 2 Patient Enrollment Form#N#Download the Patient Enrollment Form using the link below.#N#Select "Copay Assistance Program for PERSERIS" and complete the required steps outlined on page 1. Please ensure that the signature and date are provided on both the Provider Attestation and Patient Authorizations.#N#Fax the completed form directly to INSUPPORT® at 1-833-404-4897.
Can you get copay assistance with Medicare?
Patients with government insurance are not eligible for the Copay Assistance Program, including, but not limited to, Medicare, Medicaid, Medigap, VA, DoD, TRICARE, CHAMPVA, or any other federally or state-funded government-assisted program. Other restrictions apply.
Does Insupport automatically determine eligibility?
Note that for patients enrolled in "Benefit Coverage Information", INSUPPORT® will automatically determine eligibility and enroll an eligible patient in the Copay Assistance Program for PERSERIS.
Does Insupport provide copay ID?
INSUPPORT® will provide the enrolling healthcare provider with the patient's Copay Member ID for submission of copay claims to INSUPPORT®, as well as copay claim submission instructions (this information is provided immediately if using web enrollment). A Copay Member ID card will be provided to the patient in his/her welcome letter upon enrollment in the program.
INDICATION
SUBOXONE (buprenorphine and naloxone) Sublingual Film ® (CIII) is a prescription medicine used to treat opioid addiction in adults and is part of a complete treatment program that also includes counseling and behavioral therapy.
IMPORTANT SAFETY INFORMATION
Keep SUBOXONE Sublingual Film in a secure place out of sight and reach of children, and in a location not accessible by others, including visitors to the home. Accidental use by a child is a medical emergency and can result in death. If a child accidentally takes SUBOXONE Sublingual Film, get emergency help or call 911 right away.
What are the factors that affect Indivior Group?
Various factors may cause differences between Indivior's expectations and actual results, including: factors affecting sales of Indivior Group’s products; the outcome of research and development activities; decisions by regulatory authorities regarding the Indivior Group’s drug applications ; the speed with which regulatory authorizations, pricing approvals and product launches may be achieved; the outcome of post approval clinical trials; competitive developments; difficulties or delays in manufacturing; the impact of existing and future legislation and regulatory provisions on product exclusivity ; trends toward managed care and healthcare cost containment; legislation or regulatory action affecting pharmaceutical product pricing , reimbursement or access; claims and concerns that may arise regarding the safety or efficacy of the Indivior Group’s products and product candidates; risks related to legal proceedings, including the ongoing investigative and antitrust litigation matters ; the Indivior Group’s ability to protect its patents and other intellectual property; the outcome of patent infringement litigation relating to Indivior Group’s products , including the ongoing ANDA lawsuits; changes in government al laws and regulations; issues related to the outsourcing of certain operational and staff functions to third parties; uncertainties related to general economic, political, business, industry, regulatory and market conditions; and the impact of acquisitions, divestitures, restructurings, internal reorganizations, product recalls and withdrawals and other unusual items.
Is Perseris an injectable?
Slough, UK and Richmond, VA, 27 February 2019 – Indivior PLC (LON: INDV) today announced the launch of once-monthly PERSERISTM (risperidone) for extended-release injectable suspension in the United States. PERSERIS is approved by the U.S. Food and Drug Administration for the treatment of schizophrenia in adults. PERSERIS delivers its active ingredient, risperidone, in an extended-release delivery system with no loading doses or oral supplementation recommended.