Where can I use Mylotarg?
Valid at all major chains including Walgreens, CVS Pharmacy, Target, WalMart Pharmacy, Duane Reade and 65,000 pharmacies nationwide. Mylotarg offers may be in the form of a printable coupon, rebate, savings card, trial offer, or free samples.
Are prices in Mylotarg price guide valid with insurance?
Prices are for cash paying customers only and are not valid with insurance plans. This Mylotarg price guide is based on using the Drugs.com discount card which is accepted at most U.S. pharmacies.
What is the appropriate dose of cytarabine for the treatment of Mylotarg?
The cytarabine dose was 3 g/m 2 for patients less than 55 years old and 1 g/m 2 for patients 55 years or older and/or patients with a creatinine clearance below 50 mL/minute. Hematopoietic stem cell transplantation (HSCT) was allowed after treatment with MYLOTARG, but it was recommended to delay HSCT by at least 90 days following MYLOTARG.
Can Mylotarg be used in combination with chemotherapy in de novo AML?
Non-US residents click here. MYLOTARG in combination with chemotherapy was evaluated in ALFA-0701 (NCT00927498), a multicenter, randomized, open-label Phase 3 study of 271 patients with newly-diagnosed de novo AML ages 50 to 70 years.

Why was MYLOTARG removed from the market?
Mylotarg was voluntarily withdrawn from the market after subsequent confirmatory trials failed to verify clinical benefit and demonstrated safety concerns, including a high number of early deaths.
What is MYLOTARG used for?
This medication is used to treat a certain type of leukemia (CD33-positive acute myeloid leukemia-AML). This medication works by slowing or stopping the growth of cancer cells.
Is MYLOTARG approved?
On September 2, 2017, the U.S. Food and Drug Administration approved gemtuzumab ozogamicin (GO; Mylotarg; Pfizer, New York City, NY) for treatment of relapsed or refractory (R/R) CD33‐positive acute myeloid leukemia (AML) in patients 2 years of age and older.
Is MYLOTARG a chemo?
Mylotarg contains a chemotherapy drug that's attached to a monoclonal antibody. Monoclonal antibodies are drugs made from immune system cells. These drugs attach to specific proteins on cells. The monoclonal antibody in Mylotarg attaches to a specific protein that's found on cancerous cells.
What type of drug is MYLOTARG?
Mylotarg is a prescription medicine used to treat the symptoms of Acute Myeloid Leukemia. Mylotarg may be used alone or with other medications. Mylotarg belongs to a class of drugs called Antineoplastics, Antibiotic; Antineoplastics, Monoclonal Antibody.
Which cell cycle is most affected by cytarabine?
The sugar moiety of cytarabine hinders the rotation of the molecule within the DNA. The DNA replication ceases, specifically during the S phase of the cell cycle, making it a specific drug for rapidly dividing cells, such as those seen in cancer.
Why was gemtuzumab ozogamicin withdrawn?
The most common causes of treatment-related fatal adverse events were hemorrhage, infection, and/or acute respiratory distress syndrome. This data combined with other negative phase III studies led to Pfizer voluntarily withdrawing gemtuzumab ozogamicin from the market, 10 years after its original approval (NCI, 2010).
When was gemtuzumab ozogamicin approved by FDA?
On September 1, 2017, the U.S. Food and Drug Administration approved gemtuzumab ozogamicin (Mylotarg, Pfizer Inc.)
What is Gemtuzumab used for?
Descriptions. Gemtuzumab injection is used alone or together with other medicines (eg, cytarabine, daunorubicin) to treat newly-diagnosed CD33-positive acute myeloid leukemia (AML) in adults.
What is da chemotherapy?
DA – Daunorubicin and ara-C (cytarabine) – is a combination of two chemotherapy drugs. Cytarabine is also known as cytosine arabinoside (ara-C). Each works in a slightly different way and in the DA regime they are used at the optimum doses to allow the maximum effects in terms of killing cancer cells.
How does gemtuzumab ozogamicin work?
Etoposide may stop the growth of cancer cells by blocking some of the enzymes needed for cell growth. Gemtuzumab ozogamicin is a monoclonal antibody, gemtuzumab, linked to a toxic agent called ozogamicin. Gemtuzumab attaches to CD33 positive cancer cells in a targeted way and delivers ozogamicin to kill them.
Who makes Ivosidenib?
Please see Full Prescribing Information, including BOXED WARNING for AML patients and Medication Guide. TIBSOVO is a registered trademark of SERVIER PHARMACEUTICALS LLC, a wholly owned, indirect subsidiary of LES LABORATOIRES SERVIER.
What is Gemtuzumab used for?
Descriptions. Gemtuzumab injection is used alone or together with other medicines (eg, cytarabine, daunorubicin) to treat newly-diagnosed CD33-positive acute myeloid leukemia (AML) in adults.
Is Tivdak chemo?
About: Tisotumab Vedotin-tftv (Tivdak™) The antibody “calls” the immune system to attack the cell it is attached to, resulting in the immune system killing the cell. Tisotumab vedotin-tftv is a monoclonal antibody attached to a chemotherapy agent called monomethyl auristatin E (MMAE), which is a microtubule inhibitor.
How is Gemtuzumab administered?
The recommended dose of MYLOTARG is 3 mg/m2/dose (up to a maximum of one 5 mg vial) infused over a 2-hour period on Days 1, 4, and 7 in combination with DNR 60 mg/m2/day infused over 30 minutes on Day 1 to Day 3, and AraC 200 mg/m2/day by continuous infusion on Day 1 to Day 7.
What is rituximab injection?
Rituximab injection is a monoclonal antibody. Rituximab injection is used together with other medicines (eg, fludarabine cyclophosphamide) to treat chronic lymphocytic leukemia (CLL). Rituximab injection is used together with methotrexate to treat the symptoms of rheumatoid arthritis.
How many mg of Mylotarg is in a carton?
MYLOTARG (gemtuzumab ozogamicin) for injection is a white to off-white lyophilized cake or powder supplied in a carton (NDC 0008-4510-01) containing one 4.5 mg single-dose vial...
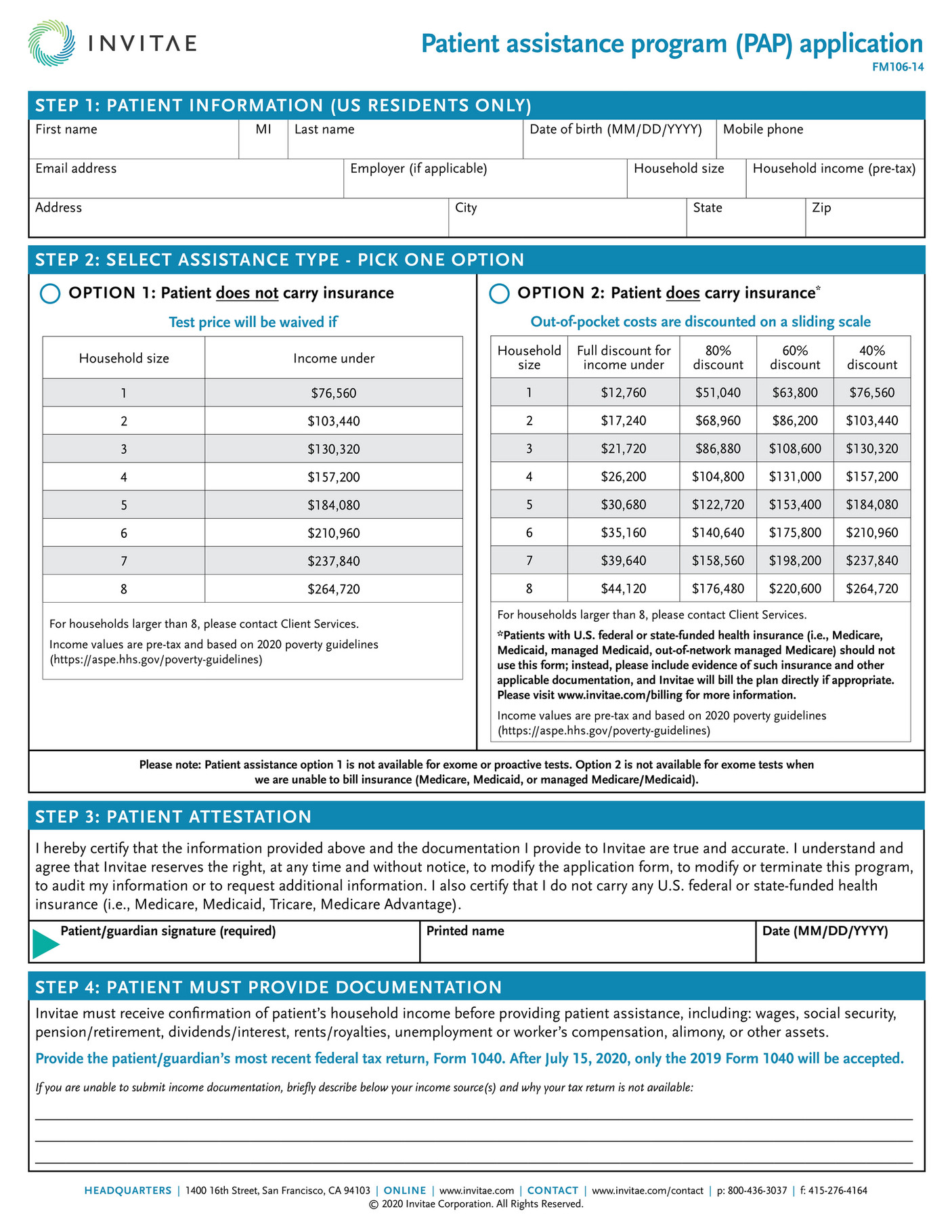
What is Pfizer Rxpathways?
Pfizer RxPathways connects eligible patients to a range of assistance programs that offer insurance support, co-pay help, and medicines for free or at a savings. Patients and physicians can contact RxPathways at (866) 706-2400 or visit the website for more information on these programs www.pfizerrxpathways.com.
Why do we need to know more about you?
In order to provide you with relevant and meaningful content we need to know more about you.
Is Mylotarg contraindicated?
MYLOTARG is contraindicated in patients with a history of hypersensitivity to the active substance in MYLOTARG or any of its components or to any of the excipients. Reactions have included anaphylaxis...
What is the Pfizer Rxpathways number?
Patients and physicians can contact RxPathways at (866) 706-2400 or visit the website for more information on these programs www.pfizerrxpathways.com.
Is Mylotarg a single agent?
The efficacy of MYLOTARG as a single agent was evaluated in MyloFrance-1 a phase 2, single-arm, open-label study in adults with CD33-positive AML in first relapse. Patients with secondary leukemia or a prior autologous or allogeneic stem cell transplantation were excluded. Study treatment included a single course of MYLOTARG 3 mg/m 2 on Days 1, 4, and 7. Consolidation therapy consisted of cytarabine intravenously every 12 hours for 3 days. The cytarabine dose was 3 g/m 2 for patients less than 55 years old and 1 g/m 2 for patients 55 years or older and/or patients with a creatinine clearance below 50 mL/minute. Hematopoietic stem cell transplantation (HSCT) was allowed after treatment with MYLOTARG, but it was recommended to delay HSCT by at least 90 days following MYLOTARG.